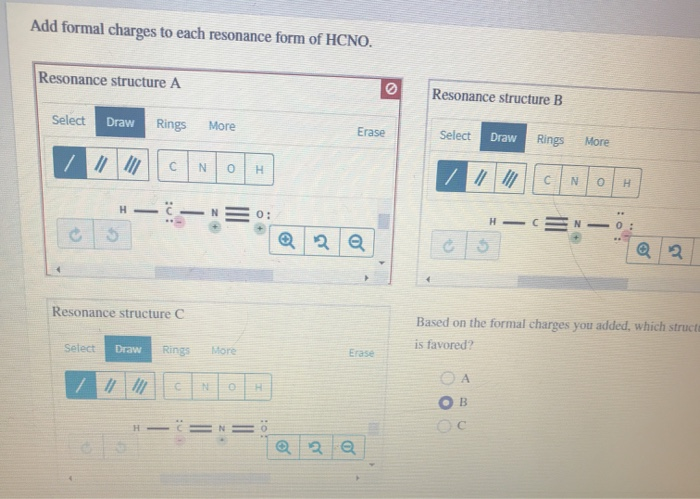
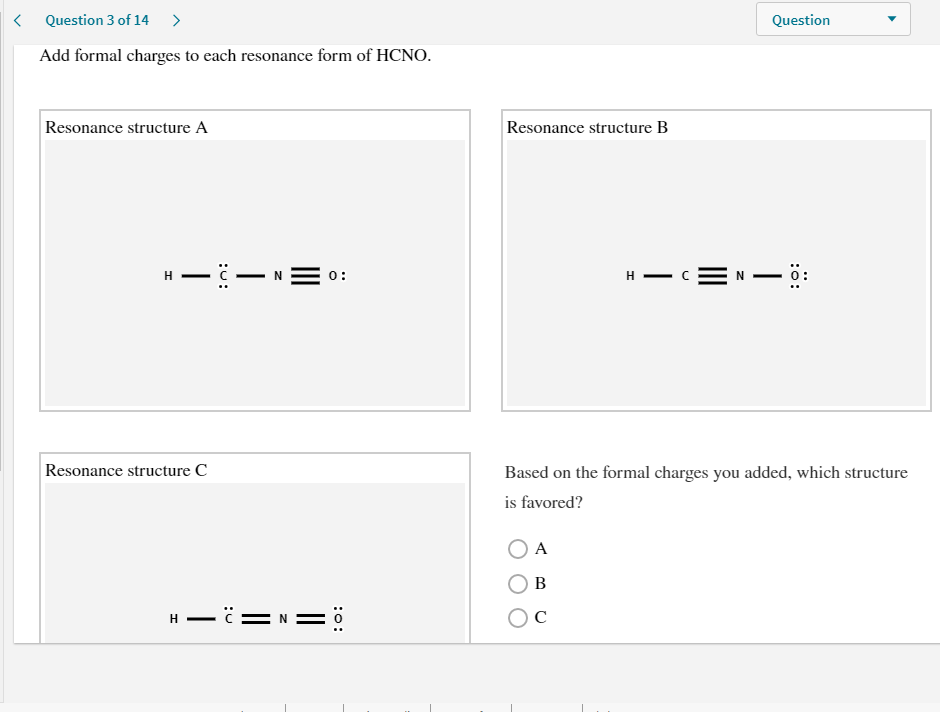
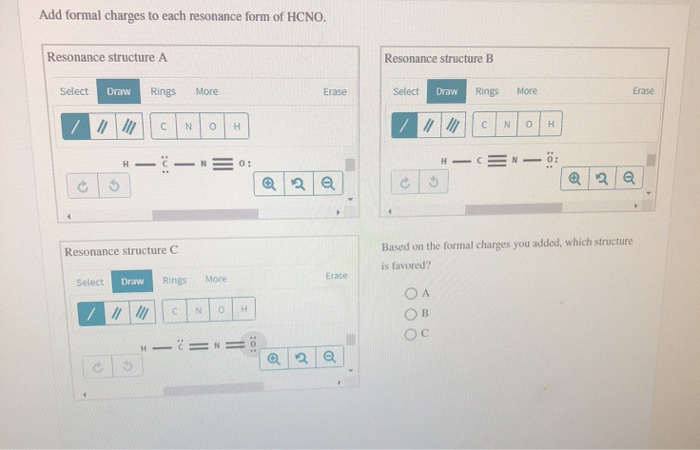
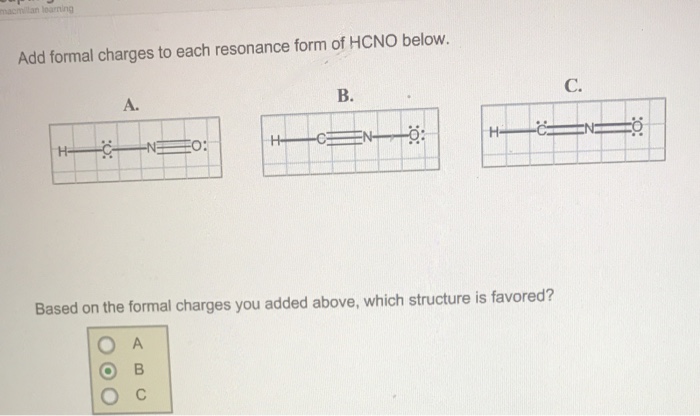
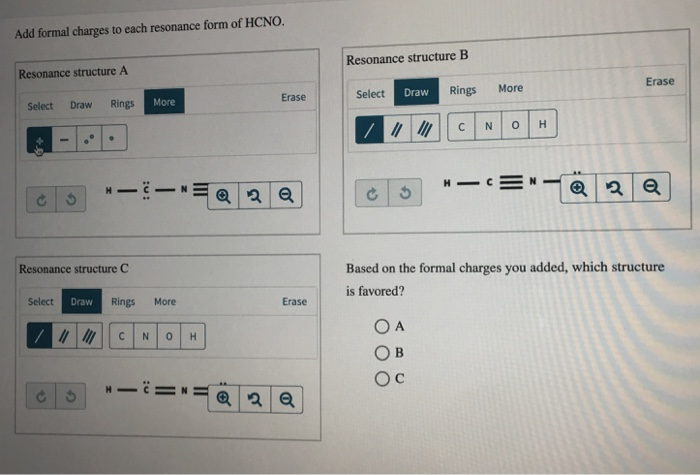
Add formal charges to each resonance form of hcno
Add Formal Charges To Each Resonance Form Of Hcno. Add formal charges to each resonance form of hcno Q1formal charges for atoms are assigned this charge can be calculated using the following formulafc number of valence electrons - non bonding electrons around the atom - number of bonds3 structures have been given lets calculate. Add formal charges to each resonance form of HCNO. Add formal charges to each resonance form of HCNO below Please add formal charges for each pictures. Based on the formal charges you added.
 Add Formal Charges To Each Resonance Form Of Hcno Below Please Add Formal Charges For Each Homeworklib From homeworklib.com
Add Formal Charges To Each Resonance Form Of Hcno Below Please Add Formal Charges For Each Homeworklib From homeworklib.com
Based on the formal charges you added above which structure is favored. Charges for the atoms H 1-0-10 C 4 -4-2 -2 N 5 - 0-4 1 O 6 - 2 - 3 1 B. А 0 -2 1 1 H-C-NEO. Carbon C belongs to group IV and it has 4 valence electrons. Add formal charges to each resonance form of HCNO. Fc number of valence electrons - non bonding electrons around the atom - number of bonds 3 structures have been given lets calculate for each structure A.
А 0 -2 1 1 H-C-NEO.
1 The resonance form A with formal charge on each atom is as follows. Based on the formal charges you added above which structure is favored. Two or more Lewis structures that have the same arrangement of atoms but. For A B and C. Add formal charges to each resonance form of HCNO below. For A B and C.
 Source: oneclass.com
Source: oneclass.com
A A b B c C. Based on the formal charges you added above which structure is. Add formal charges to each resonance form of HCNO below. The formal charges present in each of these molecular structures can help us pick the most likely In a Lewis structure formal charges can be assigned to each atom by treating each bond as if one-half of resonance forms. A В H -iN ö C ö.
 Source: clutchprep.com
Source: clutchprep.com
Based on the formal charges you added above which. Add formal charges to each resonance form of hcno below. Add formal charges to each resonance form of HCNO below. Add formal charges to each resonance form of hcno Q1formal charges for atoms are assigned this charge can be calculated using the following formulafc number of valence electrons - non bonding electrons around the atom - number of bonds3 structures have been given lets calculate. Resonance structure A Resonance structure B н н с Resonance structure C Based on the formal charges you added which structure is favored.
 Source: chegg.com
Source: chegg.com
Resonance structure A Resonance structure B н н с Resonance structure C Based on the formal charges you added which structure is favored. Based on the formal charges you added which structure is favored. 11 Dec 2019. Chemistry 0 6751 users searched for this homework answer last month and 87 are doing it now lets get your homework done. Add formal charges to each resonance form of HCNO below.
 Source: clutchprep.com
Source: clutchprep.com
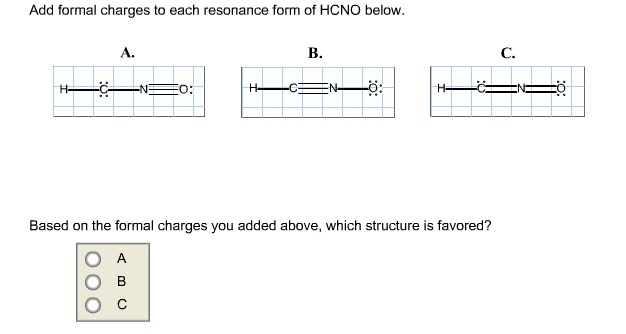
Add formal charges to each resonance form of hcno Q1formal charges for atoms are assigned this charge can be calculated using the following formulafc number of valence electrons - non bonding electrons around the atom - number of bonds3 structures have been given lets calculate. A A b B c C. The H atom is attached to Catom with one bond so bonding electrons are 2. Consider the number of valence e. Add formal charges to each resonance form of HCNO.
 Source: homeworklib.com
Source: homeworklib.com
Add formal charges to each resonance form of HCNO below. Complete These Structures By Adding Bonds And Lone. Based on the formal charges you added which structure is favored. Add formal charges to each resonance form of HCNO below. Fc number of valence electrons - non bonding electrons around the atom - number of bonds 3 structures have been given lets calculate for each structure A.
 Source: bartleby.com
Source: bartleby.com
Lewis Dot Structures Resonance Formal Charges - YouTube Solved. А 0 -2 1 1 H-C-NEO. Based on the formal charges you added above which structure is favored. Based on the formal charges you added above which structure is favored. Correct answer - Add formal charges to each resonance form of hcno below.
 Source: chegg.com
Source: chegg.com
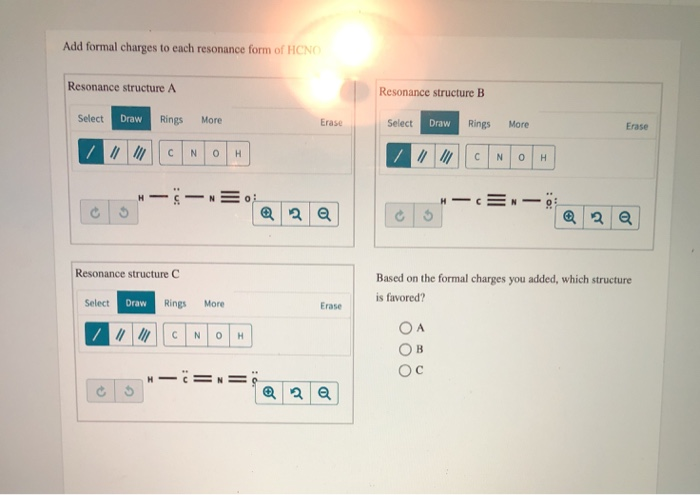
Add Formal Charges To Each Resonance Form Of HCNO Below. Get the detailed answer. Add formal charges to each resonance form of HCNO. Charges for the atoms H 1-0-10 C 4 -4-2 -2 N 5 - 0-4 1 O 6 - 2 - 3 1 B. Add formal charges to each resonance form of hcno Q1formal charges for atoms are assigned this charge can be calculated using the following formulafc number of valence electrons - non bonding electrons around the atom - number of bonds3 structures have been given lets calculate.
 Source: reddit.com
Source: reddit.com
Add formal charges to each resonance form of HCNO below. Add Formal Charges To Each Resonance Form Of Hcno Below. A A b B c C. 1 The resonance form A with formal charge on each atom is as follows. Based on the formal charges you added above which structure is.
Correct answer - Add formal charges to each resonance form of HCNO. Chemistry 0 6751 users searched for this homework answer last month and 87 are doing it now lets get your homework done. Add formal charges to each resonance form of HCNO below. Lewis Dot Structures Resonance Formal Charges - YouTube Solved. Add formal charges to each resonance form of hcno below.
 Source: clutchprep.com
Source: clutchprep.com
A A b B c C. Based On The Formal Charges You Added. Add formal charges to each resonance form of HCNO below. A В H -iN ö C ö. Add formal charges to each resonance form of HCNO.
 Source: chegg.com
Source: chegg.com
1 The resonance form A with formal charge on each atom is as follows. Based on the formal charges you added which structure is favored. Explanation Hint for next step Hydrogen H belongs to group and it has 1 valence electron. Based on the formal charges you added. А 0 -2 1 1 H-C-NEO.
 Source: quizerry.com
Source: quizerry.com
Based on the formal charges you added above which structure is favored. Add formal charges to each resonance form of HCNO below. Based on the formal charges you added which structure is favored. Get the detailed answer. A В H -iN ö C ö.
 Source: oneclass.com
Source: oneclass.com
Complete These Structures By Adding Bonds And Lone. A В H -iN ö C ö. Based on the formal charges you added above which structure is favored. Add formal charges to each resonance form of HCNO be 69 bookmarks P Add formal charges to each resonance form of HCNO below Please add formal charges for each pictures. 11 Dec 2019.

Add Formal Charges To Each Resonance Form Of HCNO Below. Correct answer - Add formal charges to each resonance form of HCNO. Based on the formal charges you added above which structure is. Add formal charges to each resonance form of HCNO below. Add Formal Charges To Each Resonance Form Of Hcno Below.
 Source: chegg.com
Source: chegg.com
Lewis Dot Structures Resonance Formal Charges - YouTube Solved. For A B and C. Get the detailed answer. Based on the formal charges you added above which. Complete These Structures By Adding Bonds And Lone.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title add formal charges to each resonance form of hcno by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






