Bbc bitesize ionic bonding
Bbc Bitesize Ionic Bonding. It covers the areas of the Chemistry Foundatio. The melting points decrease as. A three-dimensional ball and stick model for the ionic lattice in sodium chloride An ionic lattice is held together by strong of attraction between the oppositely charged ions. Learn about and revise ionic compounds with this BBC Bitesize GCSE Combined Science Edexcel study guide.
 Bonding Chemistry Gcse Revision From revisionscience.com
Bonding Chemistry Gcse Revision From revisionscience.com
Ionic bonding Find the match. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Learn about and revise ionic compounds with this BBC Bitesize GCSE Combined Science Edexcel study guide. Atoms lose or gain electrons to attain a complete outer shell of electrons. Ionic bonding Find the match. When atoms gain electrons they become negative ions.
Metals form positive ions because they lose electrons to.
This Foundation GCSE BBC Bitesize video is from the original programmes from 2000 that were broadcast on BBC2. An ionic bond is formed when electrons are lost and gained by two or more atoms. Ionic bonding is strong and requires substantial amounts of energy to break. HttpswwwfreesciencelessonscoukworkbooksThis video is for the new GCSE specifications levels 1-9 for all exam boards. Ions form when a metal reacts with a non-metal. Ionic bonding Labelled diagram.
 Source: revisionscience.com
Source: revisionscience.com
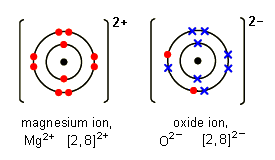
Learn about and revise ionic compounds with this BBC Bitesize GCSE Combined Science Edexcel study guide. Magnesium Mg has the electron arrangement 282. Find my revision workbooks here. Ionic bonds covalent bonds and metallic bonds are examples of chemical bonds. These are positively charged because they have fewer electrons in their shells than protons in their nuclei.
 Source: novocom.top
Source: novocom.top
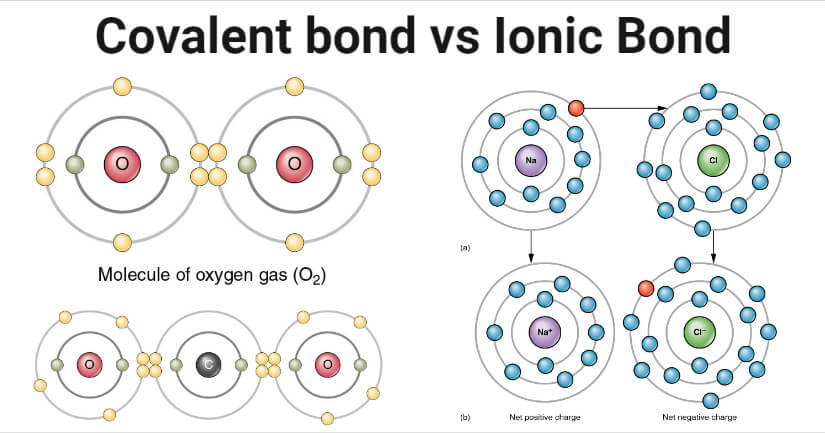
When atoms gain electrons they become negative ions. The ionic bond is the electrostatic force of attraction between a positively charged metal ion and a negatively charged non-metal ion. Learn about and revise ionic compounds with this BBC Bitesize GCSE Combined Science Edexcel study guide. Ionic bonding Find the match. The structure and bonding in a substance are modelled in different ways including dot and cross diagrams.
 Source: thechemistrynotes.com
Source: thechemistrynotes.com
Forming ionic bonds Positively charged ions are called cations and negatively charged ions are called anions. 502 results for ionic bonding. Ionic bonding Labelled diagram. These ions can form when a metal reacts with a non-metal by transferring electrons. The melting points decrease as.
 Source: chemistryrevisionforgcse.blogspot.com
Source: chemistryrevisionforgcse.blogspot.com
Positive and negative ions form when a metal reacts with a non-metal by transferring electrons. Bonding Ionic bonds covalent bonds and metallic bonds are examples of chemical bonds. The melting points decrease as. These are positively charged because they have fewer electrons in their shells than protons in their nuclei. It covers the areas of the Chemistry Foundatio.
 Source: novocom.top
Source: novocom.top
Forming ionic bonds Positively charged ions are called cations and negatively charged ions are called anions. Magnesium Mg has the electron arrangement 282. It covers the areas of the Chemistry Foundatio. The structure and bonding in a substance are modeled in different ways including dot and cross diagrams. The structure and bonding in a substance are modelled in different ways including dot and cross diagrams.
 Source: youtube.com
Source: youtube.com
Metals form positive ions because they lose electrons to. Atoms are held together by chemical bonds. Positive and negative ions form when a metal reacts with a non-metal by transferring electrons. An ionic bond is the attraction between these oppositely-charged ions. The oppositely charged ions are strongly attracted to each other forming ionic bonds.
 Source: youtube.com
Source: youtube.com
Atoms lose or gain electrons to attain a complete outer shell of electrons. Ionic bonds covalent bonds and metallic bonds are examples of chemical bonds. Everything you need to know about ionic bondingIonic bonds form when one atom transfers electrons to another atom so that both atoms forms oppositely char. The structure and bonding in a substance are modelled in different ways including dot and cross diagrams. These are positively charged because they have fewer electrons in their shells than protons in their nuclei.
 Source: revisionscience.com
Source: revisionscience.com
Atoms are held together by chemical bonds. An ionic bond is formed when electrons are lost and gained by two or more atoms. 502 results for ionic bonding. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Atoms are held together by chemical bonds.
 Source: blendspace.com
Source: blendspace.com
The melting points decrease as. Atoms lose or gain electrons to attain a complete outer shell of electrons. Magnesium Mg has the electron arrangement 282. Atoms are held together by chemical bonds. When atoms lose electrons they become positive ions.

The melting points decrease as. When atoms lose electrons they become positive ions. Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions and is the primary interaction occurring in ionic compounds. Atoms are held together by chemical bonds. Learn about and revise ionic compounds with this BBC Bitesize GCSE Combined Science Edexcel study guide.
 Source: blendspace.com
Source: blendspace.com
When atoms lose electrons they become positive ions. Atoms are held together by chemical bonds. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. An ionic bond is the attraction between these oppositely-charged ions. Magnesium Mg has the electron arrangement 282.
 Source: in.pinterest.com
Source: in.pinterest.com
The oppositely charged ions are strongly attracted to each other forming ionic bonds. Forming ionic bonds Positively charged ions are called cations and negatively charged ions are called anions. Everything you need to know about ionic bondingIonic bonds form when one atom transfers electrons to another atom so that both atoms forms oppositely char. Ionic bonding Labelled diagram. An ionic bond is the attraction between these oppositely-charged ions.
 Source: pdfprof.com
Source: pdfprof.com
Ionic bonding is strong and requires substantial amounts of energy to break. An ionic bond is the attraction between these oppositely-charged ions. They have high melting and boiling points and conduct electricity when melted or dissolved in water. A three-dimensional ball and stick model for the ionic lattice in sodium chloride An ionic lattice is held together by strong of attraction between the oppositely charged ions. The oppositely charged ions are strongly attracted to each other forming ionic bonds.
 Source: bbc.co.uk
Source: bbc.co.uk
Metals form positive ions because they lose electrons to. HttpswwwfreesciencelessonscoukworkbooksThis video is for the new GCSE specifications levels 1-9 for all exam boards. Learn about and revise ionic compounds with this BBC Bitesize GCSE Combined Science Edexcel study guide. Ionic bonds covalent bonds and metallic bonds are examples of chemical bonds. When atoms lose electrons they become positive ions.
 Source: studyrocket.co.uk
Source: studyrocket.co.uk
Ionic bonding Find the match. Ionic bonding Find the match. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The structure and bonding in a substance are modeled in different ways including dot and cross diagrams. Ionic bonds covalent bonds and metallic bonds are examples of chemical bonds.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title bbc bitesize ionic bonding by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.