Clo3 lewis structure formal charge
Clo3 Lewis Structure Formal Charge. These are some keyword suggestions for the term Lewis Structure For Clo3. Boiling Pt deg C. The Lewis structure for a chlorate ion ClO3- should show. We will now check.
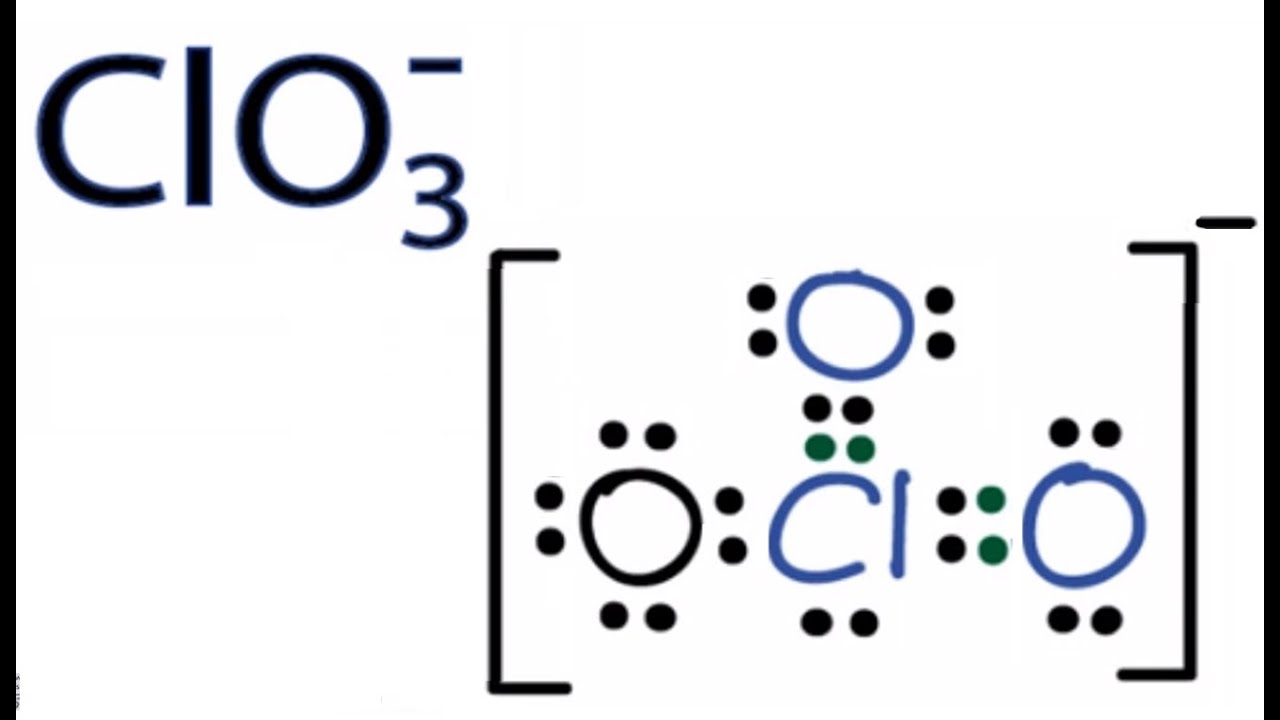 Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube From youtube.com
Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube From youtube.com
Boiling Pt deg C. Black And Tan Schipperke German Blitzkrieg In England Walking Dead Rosita Cute And Smart Baby Girls Black. Did I Do These Correctly. Which satisfies the octet rule rather well but even though the formal charges add up to -1 the charges are too spread out and so we must see if we can minimize them. So lets see what that does to our formal charges now that weve moved those in there to. In order to calculate the formal charges for ClO3- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e.
In order to calculate the formal charges for ClO3- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e.
Shown here is a Lewis structure for the chlorate ion ClO3- that obeys the octet rule showing all non-zero formal charges. Lewis Structure of ClO 3-Chlorate ion Lewis structure of ClO 3-ion is drawn step by step in this tutorial. The Lewis structure for a chlorate ion ClO3- should show. Now we should try to find a more stable structure. Draw the Lewis structure for one of the possible resonance s. The Cl has 7 valence electrons and there are 3 oxygen atoms.
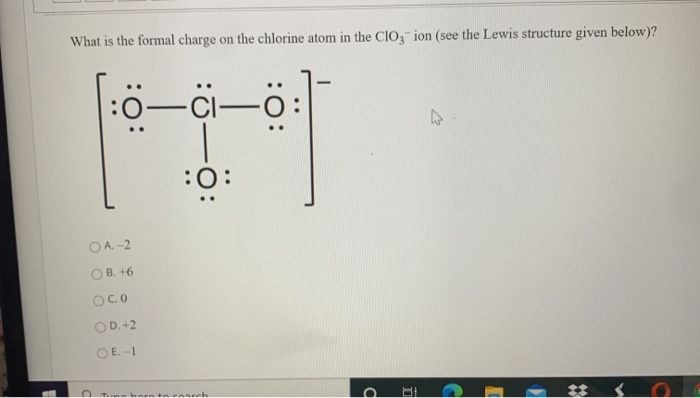 Source: chegg.com
Source: chegg.com
Total Formal Charge 0 Both Lewis structures satisfy the octet rule. Draw Lewis Dot Structure. The drawn structure for ClO 4-is not a stable structure because oxygen atoms and chlorine atoms have charges. What is formal charge. A Lewis stucture of O 2.
 Source: youtube.com
Source: youtube.com
I will tell you that in the end the Cl formal charge is 2 for ClO3-. Lewis structure 2b is preferable because there is no positive charge on Cl which is a very electronegative atom and there are no formal charges on any of the atoms formal charges are minimized. There are a total of 26 valence electrons for ClO 3-. That means that it can often hold more than 8 valence electrons so we need to check our formal charges. When we assign a charge to any bonded atom it is assumed that the charge is shared equally among all the bonded atoms.

Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for ClO3-. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for ClO 3-. Images for Clo3 Lewis Structure Formal Charge. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for ClO3-. Based on the formal charges draw the most preferred Lewis s.
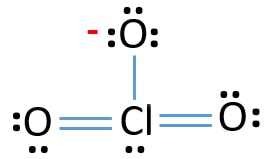 Source: chemistryscl.com
Source: chemistryscl.com
There are one chlorine atom and three oxygen atoms in the chlorate ion. The drawn structure for ClO 4-is not a stable structure because oxygen atoms and chlorine atoms have charges. What is the Lewis dot diagram for CLO3. Lewis Structure of ClO3- Chlorate ion The Predicted Stabilities of Resonance Contributors MCC. Shown here is a Lewis structure for the chlorate ion ClO3- that obeys the octet rule showing all non-zero formal charges.
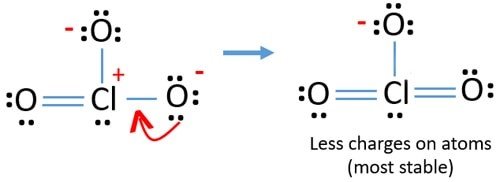 Source: geometryofmolecules.com
Source: geometryofmolecules.com
We can use 2 lone pairs from two of the O atoms to turn the single bonds of those respective atoms into double bonds and so minimize the formal charge on both O atoms and also the central Cl atom. The mixture can be ignited by friction. In order to calculate the formal charges for ClO3- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e. So lets see what that does to our formal charges now that weve moved those in there to. Asked Aug 16 2019 in Chemistry by Buggy_boy.
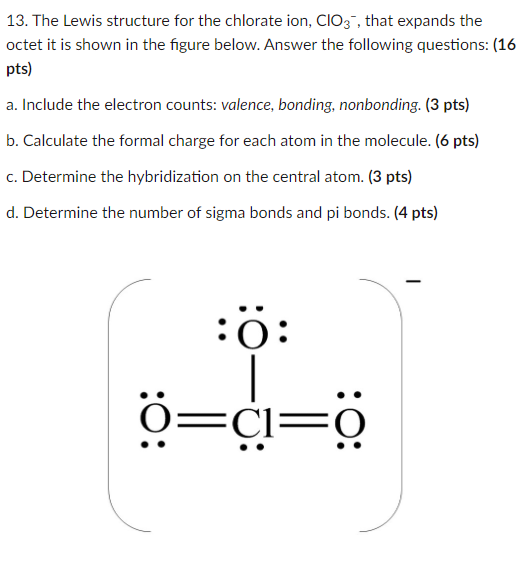 Source: chegg.com
Source: chegg.com
Total Formal Charge 0 Both Lewis structures satisfy the octet rule. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for ClO3-. When a molecule or ion has so many charges on atoms that structure is not stable. Lets take a look and see how we can fix this. What is formal charge.

Given this electronic formalism the oxygen centre is formally negative and our Lewis structure certainly represents this. A step-by-step explanation of how to draw the ClO3- Lewis Structure Chlorate Ion. What is formal charge. The charge on Cl in ClO3- is 5. When a molecule or ion has so many charges on atoms that structure is not stable.
 Source: clutchprep.com
Source: clutchprep.com
The material itself is noncombustible but it can form a very flammable mixture with combustible materials and this mixture may be explosive if the combustible material is very finely divided. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for ClO 3-. What is formal charge. We will now check. We are never really sure whether we have got our perfect Lewis structure.
 Source: youtube.com
Source: youtube.com
The ClO3- Lewis structure is. That means that it can often hold more than 8 valence electrons so we need to check our formal charges. The rules are each atom gets half of a covalent bond and all the unbonded electrons. Images for Clo3 Lewis Structure Formal Charge. You then subtract the number of electrons around the atom in the co.
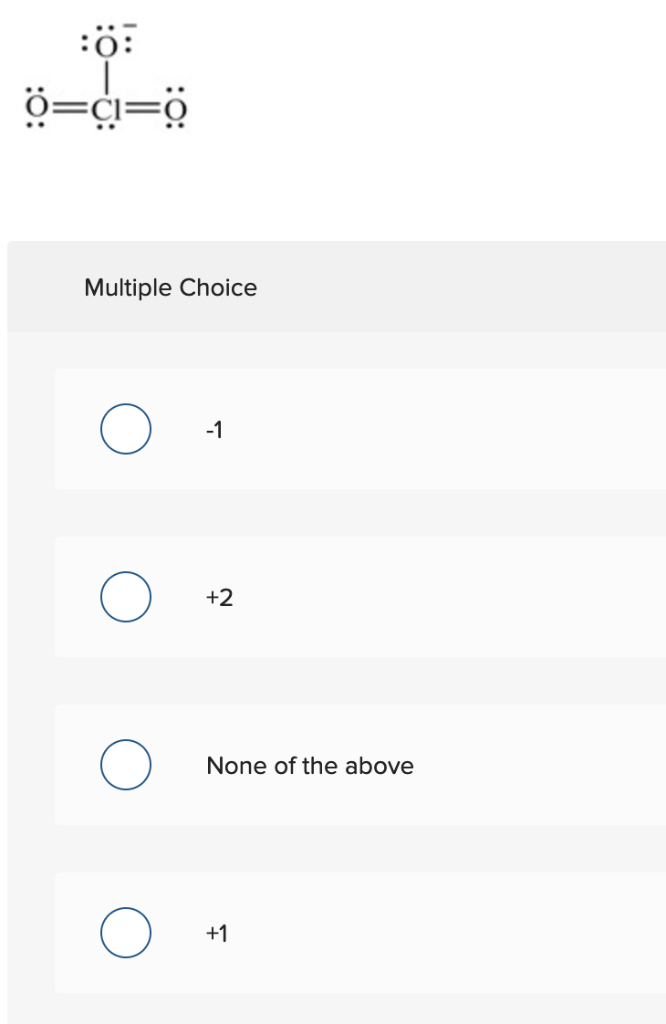 Source: chegg.com
Source: chegg.com
Lewis Structure of ClO 3-Chlorate ion Lewis structure of ClO 3-ion is drawn step by step in this tutorial. Draw Lewis Dot Structure. Draw the Lewis structure for one of the possible resonance s. We are never really sure whether we have got our perfect Lewis structure. It is soluble in waterThe material itself is noncombustible but it can form a very.
 Source: youtube.com
Source: youtube.com
Lewis Structure of ClO 3-Chlorate ion Lewis structure of ClO 3-ion is drawn step by step in this tutorial. Draw The Best Lewis Structures For The Following. Lewis structure 2b is preferable because there is no positive charge on Cl which is a very electronegative atom and there are no formal charges on any of the atoms formal charges are minimized. Valence shell expansion nyuedu. Draw the Lewis structure of ClO3- - Chemistry - Chemical.
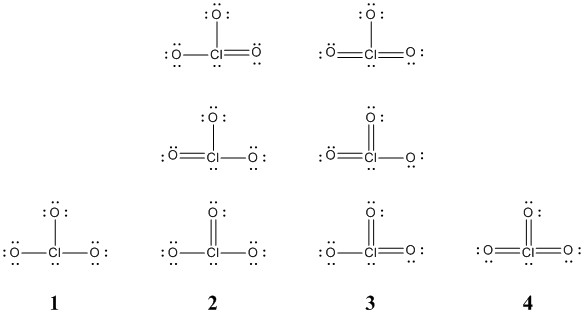 Source: chm.uri.edu
Source: chm.uri.edu
What is formal charge. We are never really sure whether we have got our perfect Lewis structure. It is soluble in waterThe material itself is noncombustible but it can form a very. That means that it can often hold more than 8 valence electrons so we need to check our formal charges. The Cl has 7 valence electrons and there are 3 oxygen atoms.
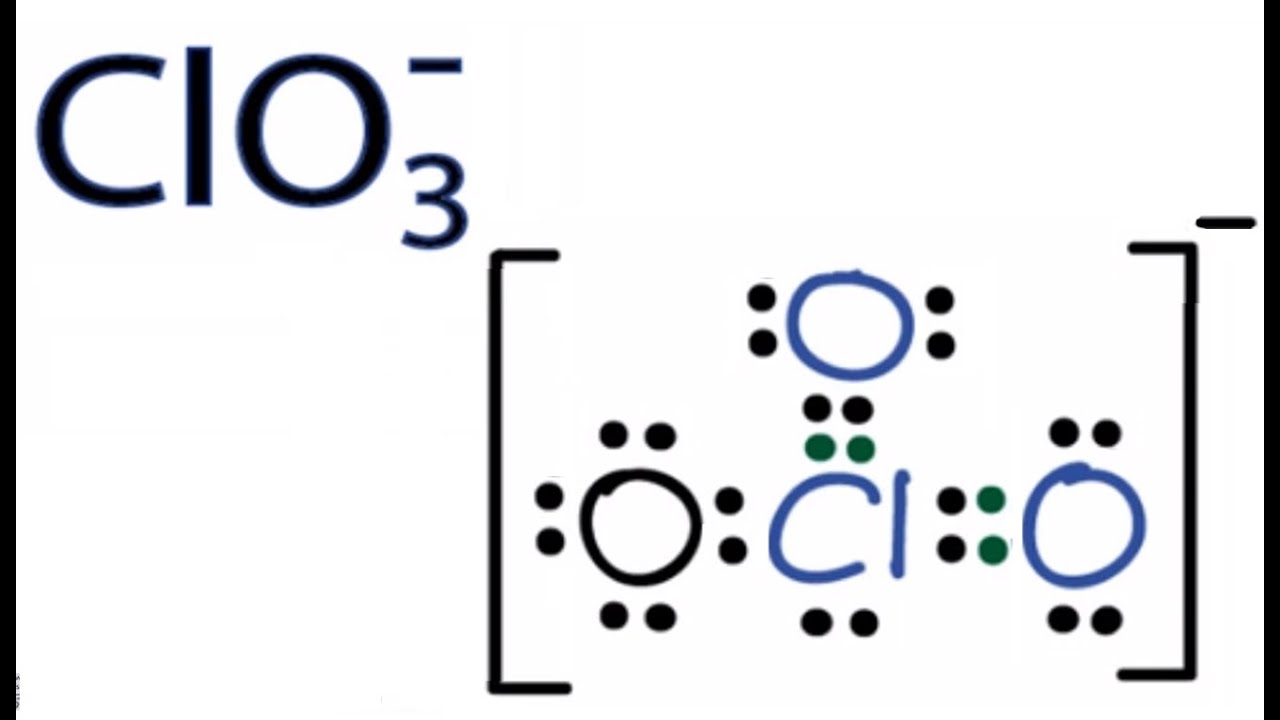 Source: youtube.com
Source: youtube.com
ClO4- Lewis Structure - How to Draw the Lewis Structure. Now we should try to find a more stable structure. A Lewis stucture of O 2. What is the Lewis dot diagram for CLO3. It is soluble in waterThe material itself is noncombustible but it can form a very.
 Source: youtube.com
Source: youtube.com
What is formal charge. The mixture can be ignited by friction. The charge on Cl in ClO3- is 5. When a molecule or ion has so many charges on atoms that structure is not stable. ClO3- Molecular Geometry Shape and Bond Angles.
 Source: quora.com
Source: quora.com
This is known as a formal charge. Images for Clo3 Lewis Structure Formal Charge. There are one chlorine atom and three oxygen atoms in the chlorate ion. Now we should try to find a more stable structure. There are a total of 26 valence electrons for ClO 3-.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title clo3 lewis structure formal charge by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





