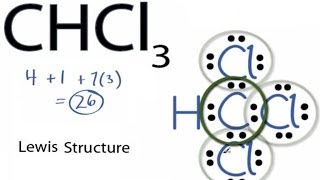
Electron dot structure chcl3
Electron Dot Structure Chcl3. Hellotoday i am going to draw the lewis structure for h2s in just four steps. Slightly more than 109 5 degrees and the hccl bond angle will then be slightly smaller why is the electron dot notation lewis structure for chcl3 what is the lewis structure diagram for chcl3 answer questions what is the net ionic equation for 2fe s 3cu no3 2 aq 3cu s 2fe no3 3 aq this is a reduction reaction so is there such a thing lewis. For the chcl3 lewis structure there are a total. Is this molecule polar or.
 Lewis Dot Structure Of K Novocom Top From novocom.top
Lewis Dot Structure Of K Novocom Top From novocom.top
The difference is that you have both Cl and F. While the IUPAC name for this molecule is trichloromethane it is more commonly known as chloroform. For the CHCl 3 Lewis structure there are a total of 26 valence electrons available. The four bonds around the central carbon atom result in a tetrahedral geometry. Photograph CHCl3 Lewis Structure. The Lewis structure for CHCl 3 is similar to CF 4 or CCl 4.
The carbon atom shares each of its four outer electrons - three with chlorine and one with hydrogen.
Each shared pair forms a single covalent bond. While the IUPAC name for this molecule is trichloromethane it is more commonly known as chloroform. The hypothetical charge that an atom would have if all bonds to atoms of different elements were fully ionic The oxidation state sometimes referred to as oxidation number describes the degree of oxidation loss of electrons of an atom in a chemical compound. The four bonds around the central carbon atom result in a tetrahedral geometry. Lewis structure electron dot diagram electron dot structure. Therefore the three Chlorine atoms contribute 7 x 3 21 valence electrons.
 Source: quora.com
Source: quora.com
Hellotoday i am going to draw the lewis structure for h2s in just four steps. Remember that Hydrogen H only needs 2 valence electrons for a full outer shell. Therefore the three Chlorine atoms contribute 7 x 3 21 valence electrons. How to Draw the Lewis Structure for. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis.
 Source: novocom.top
Source: novocom.top
The Lewis Dot Structure for CHCl3. Chlorines electronic configuration is given by Ne3s23p5. Lewis structure electron dot diagram electron dot structure. Therefore the three Chlorine atoms contribute 7 x 3 21 valence electrons. Listen carefully u knw how to make the electron dot structure of CH4.
 Source: novocom.top
Source: novocom.top
They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Draw the Lewis dot structure for CHCl3. Each chlorine contains 3. No lone pair present on the central atom of the CHCl3 lewis structure. The carbon atom shares each of its four outer electrons - three with chlorine and one with hydrogen.
 Source: topblogtenz.com
Source: topblogtenz.com
Electron Dot Structure For Chcl3. Photograph Lewis CHCl3 Janet Gray Coonce. Chcl3 electron dot structure Number that describes the degree of oxidation of an atom in a chemical compound. Lewis structure electron dot diagram electron dot structure. The chlorine atoms each share one of their 7 outer electrons with the carbon.
 Source: clutchprep.com
Source: clutchprep.com
4C 1H 21Cl 26 valence electrons. Is this molecule polar or. Now the total number of valence electrons available in CHCl3 is given by. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Draw the Lewis dot structure for CHCl3.

There are many exceptions to this rule but it should be used as a general guide for creating lewis structures. The Lewis Dot Structure for CHCl3. Each chlorine contains 3. Photograph Lewis Structure For CHCl3 Molecular Geometry. While the IUPAC name for this molecule is trichloromethane it is more commonly known as chloroform.

Determine the electron geometry and molecular shape of this molecule. Chlorines electronic configuration is given by Ne3s23p5. For the chcl3 lewis structure there are a total. Photograph CHCl3 Lewis Structure. It is same that of CH4 only replace the 3 hydrogen atom with chlorine simple hope this helps u study well.
 Source: novocom.top
Source: novocom.top
The difference is that you have both Cl and F. Photograph CHCl3 Lewis Structure. For the CHCl 3 Lewis structure there are a total of 26 valence electrons available. Determine the electron geometry and molecular shape of this molecule. Now the total number of valence electrons available in CHCl3 is given by.
 Source: youtube.com
Source: youtube.com
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. There are many exceptions to this rule but it should be used as a general guide for creating lewis structures. How to Draw the Lewis Structure for. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Draw the Lewis dot structure for CHCl3.
 Source: youtube.com
Source: youtube.com
They also display the total number of lone pairs present in each of the atoms that constitute the molecule. CHCl3 trichloromethane or chloroform is a liquid at room temperature. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Is this molecule polar or. And the h so you have 4 left over so put two dots on the top and two on the bottom so that is the lewis dot structure chcl3 ccl4 n2ci2 must show a model to teacher molecular geometry guided inquiry tables molecule lewis dot structure 1 indicate of valence e s 2 draw structure e domain ed geometry x central atom 1 draw diagram 2 name basic ed geo.
 Source: study.com
Source: study.com
No lone pair present on the central atom of the CHCl3 lewis structure. The carbon atom shares each of its four outer electrons - three with chlorine and one with hydrogen. For the chcl3 lewis structure there are a total. The chlorine atoms each share one of their 7 outer electrons with the carbon. Is this molecule polar or.
 Source: youtube.com
Source: youtube.com
Is this molecule polar or. The Lewis structure for CHCl 3 is similar to CF 4 or CCl 4. While the IUPAC name for this molecule is trichloromethane it is more commonly known as chloroform. Photograph Lewis CHCl3 Janet Gray Coonce. It is same that of CH4 only replace the 3 hydrogen atom with chlorine simple hope this helps u study well.
 Source: youtube.com
Source: youtube.com
Photograph CHCl3 Lewis Structure. Each shared pair forms a single covalent bond. Chcl3 electron dot structure Number that describes the degree of oxidation of an atom in a chemical compound. What is the lewis dot structure for chcl3. The Lewis Dot Structure for CHCl3.
 Source: pinterest.com
Source: pinterest.com
For the chcl3 lewis structure there are a total. How to Draw the Lewis Structure for. Draw the Lewis dot structure for CHCl3. The carbon atom shares each of its four outer electrons - three with chlorine and one with hydrogen. The difference is that you have both Cl and F.
 Source: youtube.com
Source: youtube.com
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. For the CHCl 3 Lewis structure there are a total of 26 valence electrons available. Therefore the three Chlorine atoms contribute 7 x 3 21 valence electrons. Draw the Lewis dot structure for CHCl3. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electron dot structure chcl3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.