Examples of single replacement reactions in everyday life
Examples Of Single Replacement Reactions In Everyday Life. All elements undergo displacement reactions so that includes both metals and non-metals. Be as specific as you can Weir. How is single displacement used in everyday life. The general formula for this reaction is.
 Single Displacement Reaction Wikipedia From en.wikipedia.org
Single Displacement Reaction Wikipedia From en.wikipedia.org
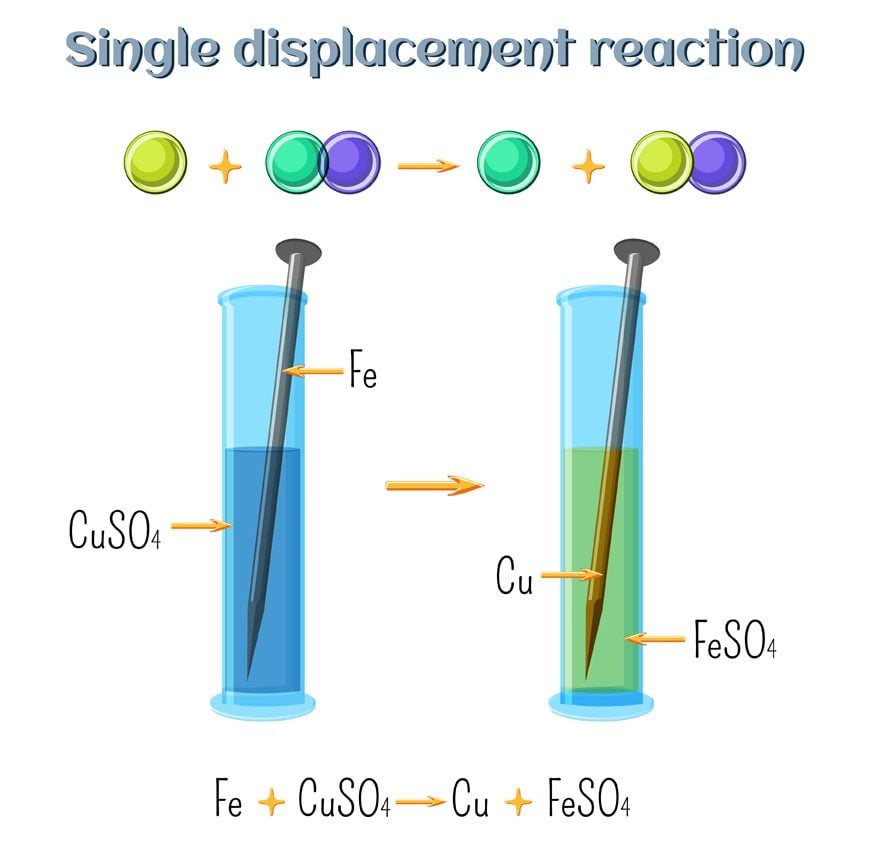
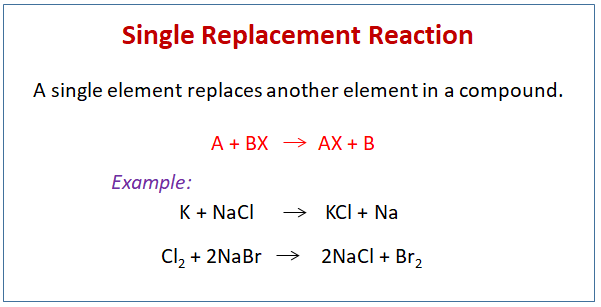
Examples of Displacement Reactions Examples of single displacement reaction Reaction between zinc and copper sulphate A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound. A BX – AX B. A B-C B A-C. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above. When this happens a new compound is formed and an element is released. A far more general definition for a displacement reaction is when a more reactive species replaces a less reactive species.
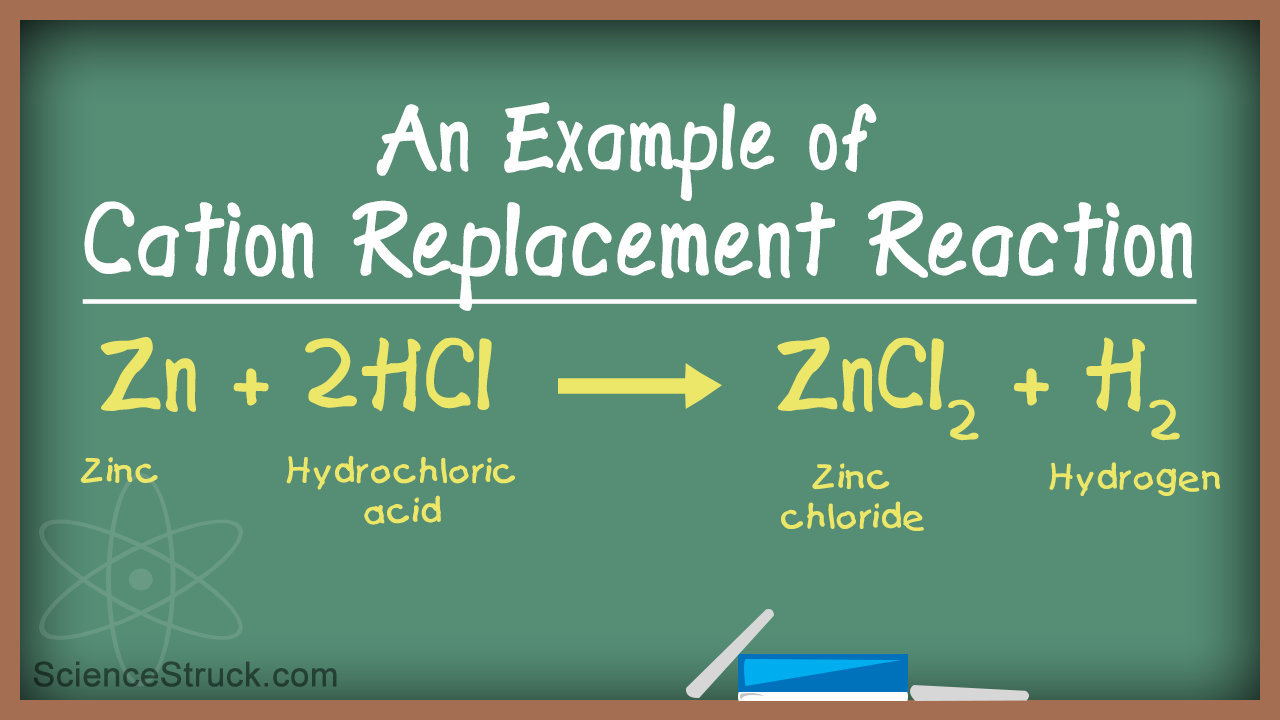
Some examples of single replacement reactions would include Zn H_2SO_4 - ZnSO_4 H_2 Zinc metal reacting in sulfuric acid to release hydrogen gas.
Here are some examples of single replacement reactions. A single replacement reaction occurs when one substance replaces another in a chemical reaction. Sodium chloride is table salt. A B-C B A-C. Suppose the solid iron III oxide were replaced with aqueous iron III chloride. For example A-B C A-C B.
 Source: chemistrylearner.com
Source: chemistrylearner.com
When calcium chloride reacts with sodium the result is sodium chloride and calcium. A colorless solid compound named potassium hydroxide KOH forms and hydrogen gas H 2 is set free. When calcium chloride reacts with sodium the result is sodium chloride and calcium. For example potassium is more reactive than magnesium so potassium replaces magnesium from magnesium chloride. A far more general definition for a displacement reaction is when a more reactive species replaces a less reactive species.
 Source: en.wikipedia.org
Source: en.wikipedia.org
I urge you to use this information to find your own examples. A far more general definition for a displacement reaction is when a more reactive species replaces a less reactive species. When this happens a new compound is formed and an element is released. Suppose the solid iron III oxide were replaced with aqueous iron III chloride. The reaction between potassium and magnesium chloride occurs as follows.
 Source: youtube.com
Source: youtube.com
Examples of Displacement Reactions Examples of single displacement reaction Reaction between zinc and copper sulphate A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound. How is single displacement used in everyday life. For example potassium is more reactive than magnesium so potassium replaces magnesium from magnesium chloride. Single replacement reactions produce a new compound and an element as products. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above.
 Source: scienceabc.com
Source: scienceabc.com
Examples of Displacement Reactions Examples of single displacement reaction Reaction between zinc and copper sulphate A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound. Real life examples of single replacement reactions include the exterior of the Statue of Liberty and processes in the steel industry. How is single displacement used in everyday life. The reaction between potassium and magnesium chloride occurs as follows. Zinc hydrochloric acid — zinc chloride and hydrogen gas zinc silver nitrate — zinc nitrate and silver metal calcium water — calcium hydroxide and dihydrogen.
 Source: study.com
Source: study.com
In these reactions more reactive metal displaces less reactive metal from its salt. Chemical Reactions in Everyday Life Web log post. Chemical reactions occur everywhere in the world around you not just in a chemistry lab. Another common type of single-replacement reaction involves a neutral metal reacting with an aqueous metal salt. The two reactions above are examples of single-replacement reactions.
 Source: prezi.com
Source: prezi.com
A single replacement reaction occurs when one substance replaces another in a chemical reaction. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above. The general formula for this reaction is. A BX – AX B. An example of a displacement reaction is.
 Source: socratic.org
Source: socratic.org
A single replacement reaction occurs when one substance replaces another in a chemical reaction. 2K 2H 2 O 2KOH H 2. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above. A common single replacement reaction occurs when zinc and copper move from aquous solutions to solids in batteries after electrons to create power. Generally metals and its salts give single displacement reactions.
 Source: sciencenotes.org
Source: sciencenotes.org
Examples of Displacement Reactions Examples of single displacement reaction Reaction between zinc and copper sulphate A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above. Chemical Reactions in Everyday Life Web log post. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above. Example 1 The extraction of magnesium from seawater MgCl2 2NaOH Mg OH2 2NaCl This chemical reaction helps purify our drinking water with little pollution.
 Source: onlinemathlearning.com
Source: onlinemathlearning.com
For example potassium is more reactive than magnesium so potassium replaces magnesium from magnesium chloride. A single replacement reaction occurs when one substance replaces another in a chemical reaction. When this happens a new compound is formed and an element is released. A far more general definition for a displacement reaction is when a more reactive species replaces a less reactive species. Chemical Reactions in Everyday Life Web log post.
 Source: sciencestruck.com
Source: sciencestruck.com
A common single replacement reaction occurs when zinc and copper move from aquous solutions to solids in batteries after electrons to create power. Examples of Displacement Reactions Examples of single displacement reaction Reaction between zinc and copper sulphate A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound. This type of a reaction can be depicted in the following manner. Generally metals and its salts give single displacement reactions. Another common type of single-replacement reaction involves a neutral metal reacting with an aqueous metal salt.
 Source: khanacademy.org
Source: khanacademy.org
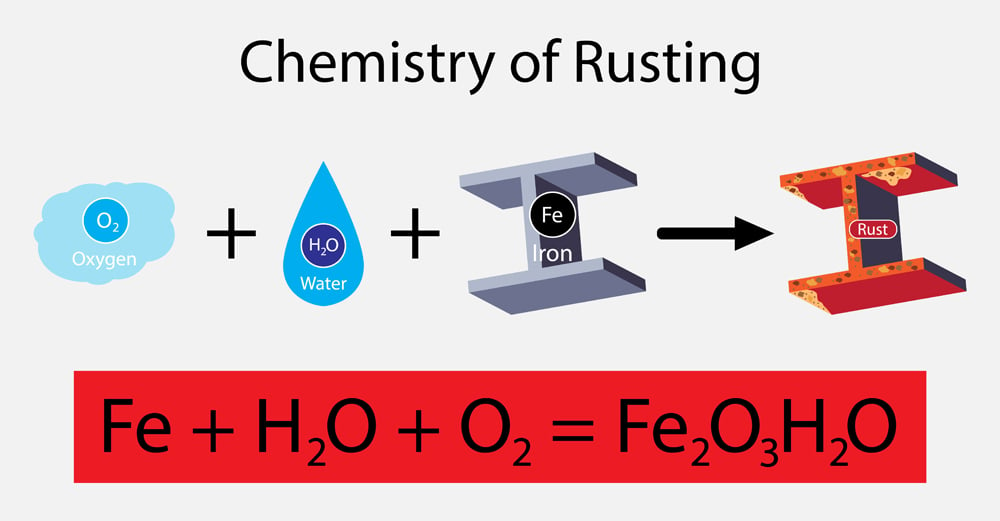
Be as specific as you can Weir. Examples of chemical reactions in everyday life include photosynthesis rust baking digestion combustion chemical batteries fermentation and washing with soap and water. Suppose the solid iron III oxide were replaced with aqueous iron III chloride. A far more general definition for a displacement reaction is when a more reactive species replaces a less reactive species. Zinc hydrochloric acid — zinc chloride and hydrogen gas zinc silver nitrate — zinc nitrate and silver metal calcium water — calcium hydroxide and dihydrogen.
 Source: ck12.org
Source: ck12.org
A common single replacement reaction occurs when zinc and copper move from aquous solutions to solids in batteries after electrons to create power. 2K 2H 2 O 2KOH H 2. A BX – AX B. Carbon dioxide and water are produced as this reaction occurs and these products are expelled through the tailpipe into the atmosphere. Single replacement reactions produce a new compound and an element as products.
 Source: scienceabc.com
Source: scienceabc.com
Chemical reactions occur everywhere in the world around you not just in a chemistry lab. For example A-B C A-C B. Example 1 The extraction of magnesium from seawater MgCl2 2NaOH Mg OH2 2NaCl This chemical reaction helps purify our drinking water with little pollution. Generally metals and its salts give single displacement reactions. Single replacement reactions produce a new compound and an element as products.
 Source: chemistrylearner.com
Source: chemistrylearner.com
A colorless solid compound named potassium hydroxide KOH forms and hydrogen gas H 2 is set free. For each of the other different type of chemical reactions double replacement single replacement composition decomposition describe a real life application in a similar manner as I have done above. Chemical reactions occur everywhere in the world around you not just in a chemistry lab. Some examples of single replacement reactions would include Zn H_2SO_4 - ZnSO_4 H_2 Zinc metal reacting in sulfuric acid to release hydrogen gas. A colorless solid compound named potassium hydroxide KOH forms and hydrogen gas H 2 is set free.
 Source: thoughtco.com
Source: thoughtco.com
The reaction between potassium and magnesium chloride occurs as follows. A BX – AX B. Sodium chloride is table salt. Examples of chemical reactions in everyday life include photosynthesis rust baking digestion combustion chemical batteries fermentation and washing with soap and water. Single replacement reactions produce a new compound and an element as products.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title examples of single replacement reactions in everyday life by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.