Lewis dot structure for ccl4
Lewis Dot Structure For Ccl4. The bond angle between the atoms is somewhere around 109 degrees. To draw the CCl4 Lewis structure carbon tetrachloride we have to find out the CC4 valence electrons firstWe express valence electrons as dots in lewis dot structure. Four dashes are drawn one on each side each connecting to a cl atom. As an example lets use carbon tetrachloride CCl4.
 6 03 Draw The Lewis Structure For Ccl4 Youtube From youtube.com
6 03 Draw The Lewis Structure For Ccl4 Youtube From youtube.com
The bond angle between the atoms is somewhere around 109 degrees. Try to draw the BrF3 Lewis structure before watching the video. CCl4 lewis structure contains four chlorine atoms and one carbon atom connected with 4 single bonds. Going from left to right there are 6 electrons around O hence neutral. How_to_draw_lewis_dot_structure_for_ccl4 24 How To Draw Lewis Dot Structure For Ccl4 DOC How To Draw Lewis Dot Structure For Ccl4 U Can. Carbon is in group 4 or 14 so it has 4.
The lewis dot structure for ccl4 starts with a c in the middle.
The nitrate ion lewis structures. Lewis Dot Structure for CCl4. Chlorine has 7 valence electrons but we have 4 Chlorines so lets multiply that by 4. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. As there are four molecules of Chlorine we will calculate the number of valence electrons accordingly. If you get the arrangement wrong you will either make a different molecule a structural iso.
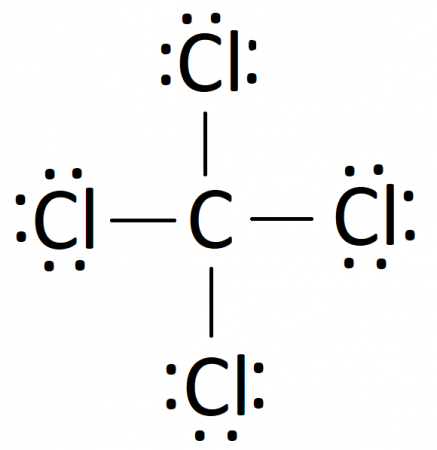 Source: sciencetrends.com
Source: sciencetrends.com
10 Pcl4 Lewis Structure. As an example lets use carbon tetrachloride CCl4. Is this molecule polar or nonpolar. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. Try to draw the BrF3 Lewis structure before watching the video.
 Source: study.com
Source: study.com
Draw the Lewis dot structure for eqCCl_4 eq. Draw the Lewis dot structure for eqCCl_4 eq. The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube To figure out lewis structure do this. Lone pairs of electrons do not involve in chemical bonds and it is represented as a dot in the lewis diagram.
 Source: novocom.top
Source: novocom.top
I also go over hybridization shape and bond angle. The electrons are represented with the help of circular dots. Moore 2015-08-10 Now you can score higher in chemistry Every high school requires a course in chemistry for graduation and many universities require the course for majors in medicine engineering biology and. This diagram displays the bonds formed as well as lone pairs of electrons. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound.

The electrons are represented with the help of circular dots. Lets do the Lewis structure for CCl4 Carbon Tetrachloride sometimes just called Carbon Tet. A ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Four plus 28 equals 32 total valence electrons to work with. Mar 8 2020 - CCl4 Lewis StructureLewis Structure for CCl4 Carbon Tetrachloride Hellotoday I am going to draw the lewis Dot structure for CCl₄ in just four steps.
 Source: sciencestruck.com
Source: sciencestruck.com
Lets do the Lewis structure for CCl4 Carbon Tetrachloride sometimes just called Carbon Tet. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. The nitrate ion lewis structures. The electrons are represented with the help of circular dots. 4 47.
 Source: youtube.com
Source: youtube.com
As there are four molecules of Chlorine we will calculate the number of valence electrons accordingly. Chlorine has 7 valence electrons but we have 4 Chlorines so lets multiply that by 4. To get the valence electrons of carbonwe need to look at the electronic configuration of carbon. 4 47. Four plus 28 equals 32 total valence electrons to work with.
 Source: sciencetrends.com
Source: sciencetrends.com
A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube To figure out lewis structure do this. Chlorine has 7 valence electrons but we have 4 Chlorines so lets multiply that by 4. To draw the CCl4 Lewis structure carbon tetrachloride we have to find out the CC4 valence electrons firstWe express valence electrons as dots in lewis dot structure.
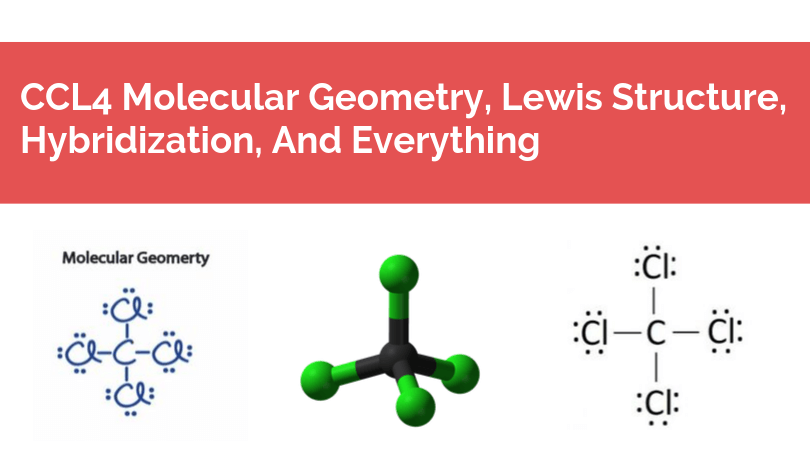 Source: geometryofmolecules.com
Source: geometryofmolecules.com
The lewis dot structure for ccl4 starts with a c in the middle. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. A ccl4 lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. 4 47. Carbon is in group 4 or 14 so it has 4.
 Source: quizlet.com
Source: quizlet.com
How_to_draw_lewis_dot_structure_for_ccl4 24 How To Draw Lewis Dot Structure For Ccl4 DOC How To Draw Lewis Dot Structure For Ccl4 U Can. The lewis structure indicates that each cl atom has three pairs of. The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. Thus the trial structure is the correct lewis structure. If you get the arrangement wrong you will either make a different molecule a structural iso.
 Source: youtube.com
Source: youtube.com
For the BrF3 Lewis structure calculate the total number of valence electrons for the BrF3. Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube To figure out lewis structure do this. Moore 2015-08-10 Now you can score higher in chemistry Every high school requires a course in chemistry for graduation and many universities require the course for majors in medicine engineering biology and. Carbon is in group 4 or 14 so it has 4. Determine the electron geometry and molecular shape of this molecule.
 Source: clutchprep.com
Source: clutchprep.com
Lets do the Lewis structure for CCl4 Carbon Tetrachloride sometimes just called Carbon Tet. Chemistry I For Dummies-John T. Chlorine has 7 valence electrons but we have 4 Chlorines so lets multiply that by 4. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. For the BrF3 Lewis structure calculate the total number of valence electrons for the BrF3.
 Source: techiescientist.com
Source: techiescientist.com
The single carbon atom contains four valence electrons and each of the four chlorine atoms contains seven valence electrons. Therefore the number of valence electrons for this molecule is 4 4 7 32. This helps us to understand the geometry of CCl4 which is tetrahedral. Try structures similar to BF3 for more practiceON-O-_2 is the standard Lewis structure. Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries.
 Source: clutchprep.com
Source: clutchprep.com
The bond angle between the atoms is somewhere around 109 degrees. The lewis dot structure for ccl4 starts with a c in the middle. The bond angle between the atoms is somewhere around 109 degrees. As an example lets use carbon tetrachloride CCl4. To get the valence electrons of carbonwe need to look at the electronic configuration of carbon.
 Source: sciencetrends.com
Source: sciencetrends.com
4 47. If you get the arrangement wrong you will either make a different molecule a structural iso. Try structures similar to BF3 for more practiceON-O-_2 is the standard Lewis structure. Is this molecule polar or nonpolar. The single carbon atom contains four valence electrons and each of the four chlorine atoms contains seven valence electrons.
 Source: youtube.com
Source: youtube.com
Determine the electron geometry and molecular shape of this molecule. Carbon is in group 4 or 14 so it has 4. To get the valence electrons of carbonwe need to look at the electronic configuration of carbon. For the Lewis structure of CCl4 first lets calculate the total valence electrons. Determine the electron geometry and molecular shape of this molecule.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis dot structure for ccl4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






