Lewis dot structure for h3po4
Lewis Dot Structure For H3po4. Here is the answer for the question Which is the Lewis structure for H3PO4. This is the h3po4 lewis structure. Therefore total number of VSPER pairs are four. Resonance In this lesson well review Lewis dot structures and how to draw them.
 How Many Sigma Bonds Are In H3po4 Quora From quora.com
How Many Sigma Bonds Are In H3po4 Quora From quora.com
Rest of all bonds are single bonds. Explain How To Determine The. Which is the lewis structure for h3po4. Resonance In this lesson well review Lewis dot structures and how to draw them. Rest of all bonds are single bonds. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules.
There are four σ bonds and no lone pairs around phosphorous atom in the lewis structure of H3PO4 molecule.
In the lewis structure of H 3 PO 4 there is one double between phosphorous atom and one oxygeen atom. 1 See answer alpandome is waiting for your help. In the h3po4 lewis structure phosphorous p is least electron electronegative atom and goes in the center of the lewis structure. Also there are no lone pairs on phosphorous atom. A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure. Which is the Lewis structure for H3PO4.
 Source: clutchprep.com
Source: clutchprep.com
Therefore shape of molecule around phosphorous atom is tetrahedral. In the h3po4 lewis structure phosphorous p is least electron electronegative atom and goes in the center of the lewis structure. Lewis dot structure of h3po4. Rest of all bonds are single bonds. Explain How To Determine The.
 Source: brainly.in
Source: brainly.in
These are some keyword suggestions for the term Lewis Dot Structure Of H3po4. Lewis dot structure of h3po4. These are some keyword suggestions for the term Lewis Dot Structure Of H3po4. In the h3po4 lewis structure phosphorous p is least electron electronegative atom and goes in the center of the lewis structure. The answer would have to be b because it is the only one that has the correct number of valence electrons.
 Source: novocom.top
Source: novocom.top
In the H 3 PO 4 Lewis structure Phosphorous P is least electron electronegative atom and goes in the center of the Lewis structure. Patricia Cohen Sac Capital Fucus Thallus Sheetal Sheth The World Unseen Banana Pie Mcdonalds Hyundai Genesis Coupe 2007 Hiv In Throat Passive Aggressive Facebook Silver Chain Necklace Png Books Clipart Png Car Rear View Mirror Accessories. Here is the answer for the question Which is the Lewis structure for H3PO4. There are three O-H bonds which give some acidic charcteristics to H 3 PO 4. This is known as the VSEPR theory or Valence Shell Electron Repulsion Pair Theory.
 Source: youtube.com
Source: youtube.com
A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure. Also there are no lone pairs on phosphorous atom. Lewis dot structure of h3po4. This is the h3po4 lewis structure. Therefore total number of VSPER pairs are four.
 Source: brainly.com
Source: brainly.com
A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure. This means that the hydrogen atoms will be. Therefore total number of VSPER pairs are four. Which is the lewis structure for h3po4. I quickly take you through how to draw the Lewis Structure of H3PO4 Phosphoric Acid.
 Source: youtube.com
Source: youtube.com
In the lewis structure of H 3 PO 4 there is one double between phosphorous atom and one oxygeen atom. The most suitable lewis structure for H3PO4 is. Which is the lewis structure for h3po4. This is the h3po4 lewis structure. Directory of Chem Help ASAP videos.
 Source: study.com
Source: study.com
Compare the formal charges on the phosphorus atoms. This is known as the VSEPR theory or Valence Shell Electron Repulsion Pair Theory. Therefore total number of VSPER pairs are four. In the lewis structure of H 3 PO 4 there is one double between phosphorous atom and one oxygeen atom. This causes repulsion which is needed to be minimized for stability and balance.
 Source: study.com
Source: study.com
Also there are no lone pairs on phosphorous atom. Alpandome alpandome 12112020 Chemistry High School answered Which is the Lewis dot structure for H3PO4. Lewis Dot Structure of H3PO4 How to Draw Lewis Structures Class 11 Chemistry Chemical BondingQueries Solved in this videos-1 lewis structure2 lew. In the h3po4 lewis structure phosphorous p is least electron electronegative atom and goes in the center of the lewis structure. Therefore shape of molecule around phosphorous atom is tetrahedral.
 Source: brainly.com
Source: brainly.com
Here is the answer for the question Which is the Lewis structure for H3PO4. A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure Phosphoric acidFor the H3PO4 structure use the periodic table to find the total nu. 1 See answer alpandome is waiting for your help. This means that the hydrogen atoms will be. In the h3po4 lewis structure phosphorous p is least electron electronegative atom and goes in the center of the lewis structure.
 Source: study.com
Source: study.com
The most suitable lewis structure for H3PO4 is. Also there are no lone pairs on phosphorous atom. Rest of all bonds are single bonds. Which is the lewis structure for h3po4. Which is the lewis structure for h3po4.
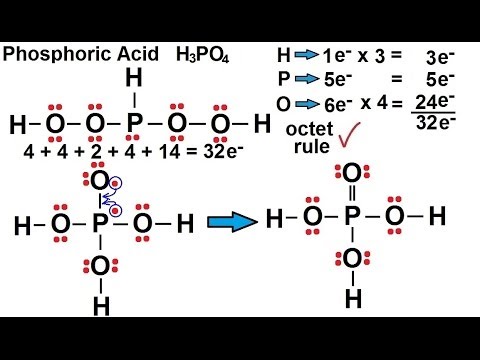 Source: youtube.com
Source: youtube.com
A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure. Which is the lewis structure for h3po4. Patricia Cohen Sac Capital Fucus Thallus Sheetal Sheth The World Unseen Banana Pie Mcdonalds Hyundai Genesis Coupe 2007 Hiv In Throat Passive Aggressive Facebook Silver Chain Necklace Png Books Clipart Png Car Rear View Mirror Accessories. In the lewis structure of H 3 PO 4 there is one double between phosphorous atom and one oxygeen atom. Alpandome alpandome 12112020 Chemistry High School answered Which is the Lewis dot structure for H3PO4.
 Source: quora.com
Source: quora.com
Lewis Dot Structure of H3PO4 How to Draw Lewis Structures Class 11 Chemistry Chemical BondingQueries Solved in this videos-1 lewis structure2 lew. Resonance In this lesson well review Lewis dot structures and how to draw them. Here is the answer for the question Which is the Lewis structure for H3PO4. This is known as the VSEPR theory or Valence Shell Electron Repulsion Pair Theory. When we have an H or H2 or H3 in front of a polyatomic molecule like CO 3 PO 4 NO 2 etc we know that its an acid.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
There are four σ bonds and no lone pairs around phosphorous atom in the lewis structure of H3PO4 molecule. There are three O-H bonds which give some acidic charcteristics to H 3 PO 4. When we have an H or H2 or H3 in front of a polyatomic molecule like CO 3 PO 4 NO 2 etc we know that its an acid. Every single bond is a sigma bond while the double bond consists of a sigma and a pi bond. Therefore total number of VSPER pairs are four.
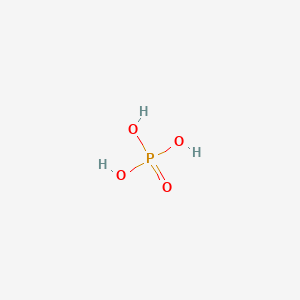
Every single bond is a sigma bond while the double bond consists of a sigma and a pi bond. I also go over hybridization shape and bond angles. Best Trick for Molecular Structure with. When we have an H or H2 or H3 in front of a polyatomic molecule like CO 3 PO 4 NO 2 etc we know that its an acid. There are three O-H bonds which give some acidic charcteristics to H 3 PO 4.
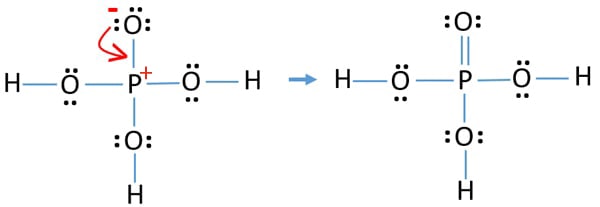 Source: chemistryscl.com
Source: chemistryscl.com
This is known as the VSEPR theory or Valence Shell Electron Repulsion Pair Theory. Which is the lewis structure for h3po4. Which is the Lewis structure for H3PO4. This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules. Which is the lewis structure for h3po4.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis dot structure for h3po4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






