Lewis dot structure for ocl2
Lewis Dot Structure For Ocl2. So we have 2 4 6 8 10 12 14. CL6-O4-CL6 Choose the best electron-dot structure for CH2Cl2. Attach the O to the Si with a double bond with 4 electrons. Place the left most atom on the periodic table in the center and the other atoms top bottom left or right.

B Carbon atoms can bond with different atoms to. Lewis structure is very important in chemistry because they are used in many important concepts of general chemistry such as chemical bonding resonance valence shell we can learn to make accurate lewis dot structures in 4 simple steps. The gas is toxic and narcotic in low concentrations and presents a moderate fire hazard. This is the OCl2 Lewis structure. B In a Lewis structure a covalent bond can be represented by a pair of electrons or a dash. Lewis Structure of Dichlorine Monoxide OCl2 Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms.
In respect to this what is the shape of OCl2.
Lewis Dot Structure and Polarity. How many single bonds are in 427 ml of sulfur trioxide at stp For each row in the table below decide whether the pair of elements wil. We can reduce the number of formal charges by moving a lone pair of electrons from o to form a clo double bond. Draw the Lewis structure for OCl2 and use it to identify the correct statements that follow. Put oxygen in center and arrange chlorine atoms on the sidesArrange electrons until both atoms get 8 electrons. These structures are depicted by the dot diagram and that dot represents the sharing of electrons between.
 Source: youtube.com
Source: youtube.com
Quick dot structure for the ammonium cation so nh 4 plus the first thing you do is find the total electrons in our dot structure for sulfuric acid all right next thing we do is choose the central lone pairs of electrons on this. ReadDownload File Report Abuse. Lewis Dot Structure and Polarity. 27 Sigma and pi bonds All single bonds are referred to as sigma bonds σ-bonds. For the OCl2 Lewis structure calculate the total number of valence electrons for the OCl2 molecule OCl2 has 20 valence electrons.
 Source: clutchprep.com
Source: clutchprep.com
SO 3 SO 2 CH 4 SF4 PCl5 IF5 N H H H O H H H Cl H C Cl Cl Cl C H H O O C O Cl C Cl Cl Cl H H F S F F F F F. Lewis dot structure of OCl 2. A step-by-step explanation of how to draw the OCl2 Lewis Dot Structure Oxygen dichlorideFor the OCl2 structure use the periodic table to find the total nu. An automatic procedure for writing canonical forms. Lewis Dot Structure and Polarity.
 Source: youtube.com
Source: youtube.com
Posted on January 01 2016. More Lewis Dot Structures. Place single bonds between the central atom and the outer atoms Note. The structure determines how sharing of the valence electrons is taking place and whether a single double or triple bond is forming. OCl2 is polar because the two O-Cl bond dipoles dont cancel each other.

A step-by-step explanation of how to draw the Cl2O Lewis Structure Dichlorine MonoxideFor the Cl2O structure use the periodic table to find the total numb. SO 3 SO 2 CH 4 SF4 PCl5 IF5 N H H H O H H H Cl H C Cl Cl Cl C H H O O C O Cl C Cl Cl Cl H H F S F F F F F. CL6-O4-CL6 Choose the best electron-dot structure for CH2Cl2. Draw the Lewis. Lewis dot structure of OCl 2.
 Source: biochemhelp.com
Source: biochemhelp.com
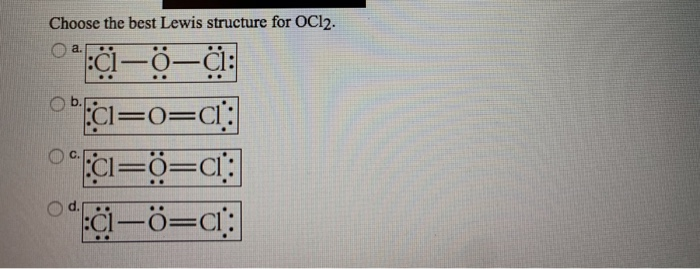
In respect to this what is the shape of OCl2. OCl2 oxygen dichloride Cl O Cl VSEPR geometry. Electron dot diagram for chlorine ocl2 lewis structure. Lewis Structure of Dichlorine Monoxide OCl2 Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. Well put two valence electrons between atoms to form chemical bonds and then well go around the outside of the Chlorines until we fill their octets or we use 20 valence electrons.
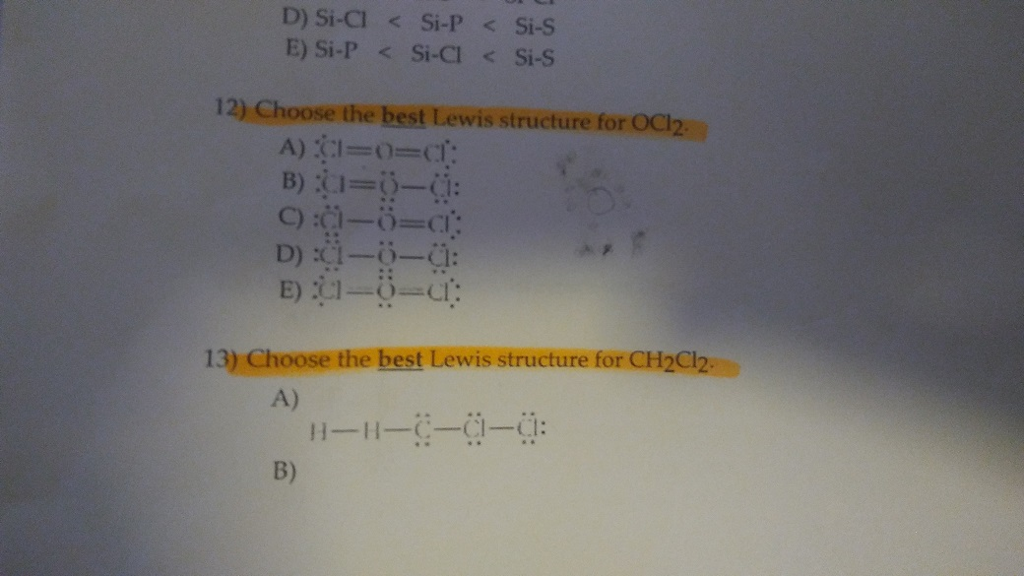 Source: chegg.com
Source: chegg.com
Electron dot diagram for chlorine ocl2 lewis structure. B In a Lewis structure a covalent bond can be represented by a pair of electrons or a dash. Draw the Lewis. Draw the dot diagram of OCl2 1. Place the left most atom on the periodic table in the center and the other atoms top bottom left or right.
 Source: novocom.top
Source: novocom.top
Well put two valence electrons between atoms to form chemical bonds and then well go around the outside of the Chlorines until we fill their octets or we use 20 valence electrons. D Si-Cl Si-P Si-S E Si-P Si-Cl Si-S 12 Choose the best Lewis structure for OCl2 D Ci– 13 Choose the best Lewis structure for CH2Cl2 A B. Which of the following is true. Calculate the total valence electrons in the molecule. The Lewis structure is proposed by the renowned scientist named as Gilbert NLewis.
 Source: study.com
Source: study.com
Calculate the total valence electrons in the molecule. Lewis Structure of Dichlorine Monoxide OCl2 Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. A step-by-step explanation of how to draw the Cl2O Lewis Structure Dichlorine MonoxideFor the Cl2O structure use the periodic table to find the total numb. ReadDownload File Report Abuse. These structures are depicted by the dot diagram and that dot represents the sharing of electrons between.
 Source: youtube.com
Source: youtube.com
B In a Lewis structure a covalent bond can be represented by a pair of electrons or a dash. Well put two valence electrons between atoms to form chemical bonds and then well go around the outside of the Chlorines until we fill their octets or we use 20 valence electrons. Use information from step 4 and 5 to draw the lewis structure. Oxygen is the least electronegative. Lewis Structure Of Ocl2 Free PDF eBooks.
 Source: youtube.com
Source: youtube.com
Dichlorine monoxide is an inorganic compound with the molecular formula Cl 2 O. Which of the following substances contains a nonpolar covalent bond. 14 6 20 total valence electrons 2. So we have 2 4 6 8 10 12 14. A step-by-step explanation of how to draw the OCl2 Lewis Dot Structure Oxygen dichlorideFor the OCl2 structure use the periodic table to find the total nu.
 Source: quizlet.com
Source: quizlet.com
Dichlorine monoxide is an inorganic compound with the molecular formula Cl 2 O. Electron dot diagram for chlorine ocl2 lewis structure. Dichlorine monoxide is an inorganic compound with the molecular formula Cl 2 O. Calculate the total valence electrons in the molecule. The oxygen has 6 valence electrons.
 Source: chegg.com
Source: chegg.com
Additionally what is the molecular shape of CO. Draw the Lewis structure for OCl2 and use it to identify the correct statements that follow. Alternatively a dot method can be used to draw the lewis structure. Which of the following substances contains a nonpolar covalent bond. These structures are depicted by the dot diagram and that dot represents the sharing of electrons between.
 Source: techiescientist.com
Source: techiescientist.com
Alternatively a dot method can be used to draw the lewis structure. B Carbon atoms can bond with different atoms to. HCl hydrochloric acid H Cl. Ocl2 lewis structure. Choose the best Lewis structure for OCl2.
 Source: clutchprep.com
Source: clutchprep.com
Indicate the VSEPR geometry about the central atom. The structure determines how sharing of the valence electrons is taking place and whether a single double or triple bond is forming. These structures are depicted by the dot diagram and that dot represents the sharing of electrons between. OCl2 is polar because the two O-Cl bond dipoles dont cancel each other. How to draw the dot structure for ocl2.
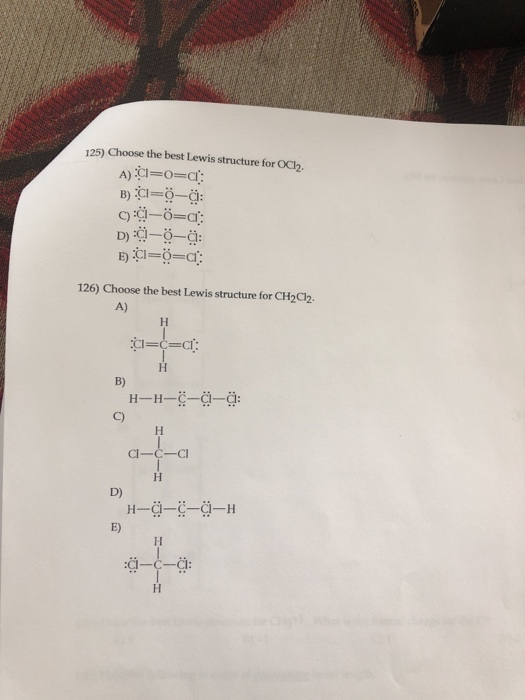 Source: chegg.com
Source: chegg.com
We can reduce the number of formal charges by moving a lone pair of electrons from o to form a clo double bond. Indicate the VSEPR geometry about the central atom. Ocl2 lewis structure. Attach the O to the Si with a double bond with 4 electrons. Well put that at the center and the Chlorines on either side.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis dot structure for ocl2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.