Lewis dot structure of bcl3
Lewis Dot Structure Of Bcl3. And put some electrons. BCl 3 Lewis Structure. 70 More Lewis Dot Structures. Use information from step 4 and 5 to draw the lewis structure.
 Bcl3 Lewis Structure How To Draw The Lewis Structure For Bcl3 Youtube From youtube.com
Bcl3 Lewis Structure How To Draw The Lewis Structure For Bcl3 Youtube From youtube.com
BCl 3 Lewis Structure. The principal quantum number of the valence electrons in a atom of lead is A. Note that Boron can have a full outershell with only six valence electrons. Lewis Dot Structure For Bcl3. 2Determine the number of electron pairs or clouds around the CENTRAL ATOM multiple bonds count as ONE CLOUD see. Molecular Geometry and Hybridization of Determine the Lewis structure.
2Determine the number of electron pairs or clouds around the CENTRAL ATOM multiple bonds count as ONE CLOUD see.
Bcl3 geometry boron trichloride lewis structure molecular dot electron bcl planar trigonal hybridization polarity draw sp octet boron trichloride bcl3 electron rule exceptions odd which molecular compound lewis dot. 70 More Lewis Dot Structures. This correlates with the property that it is dangerously reactive. I also go over formal charge hybridization shape and bond angle. Molecular Geometry and Hybridization of Determine the Lewis structure. Valence shell electron pair repulsion vsepr theory along with lewis structures can be used to predict molecular geometry.
 Source: youtube.com
Source: youtube.com
This correlates with the property that it is dangerously reactive. Lets do the Lewis structure for BCl3. 42 3-D Structure. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu. Draw the lewis structure for.
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
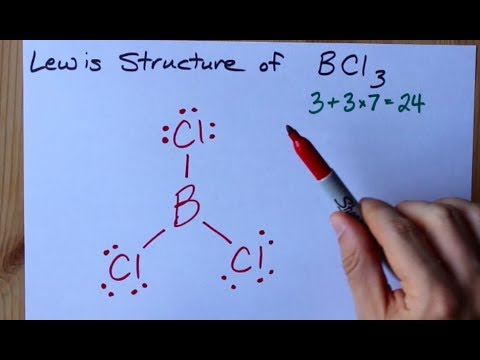
Best Lewis Dot Structure for BCl3 This is the best Lewis dot structure for BCl3From herewe see that the central atom boron does not have any lone pairs of electrons and it has six valence electronsHaving six valence electrons is not normalThus boron violates the octet rule. And we get a total of 24 valence electrons. Put atom with lowest electro negativity in the center. Boron has three valence electrons. Lewis dot structure of BCl 3.
 Source: youtube.com
Source: youtube.com
Molecular Geometry and Hybridization of Determine the Lewis structure. The Lewis dot structures of a compound represent a schematic arrangement of its constituent molecules atoms and electron bonds. If you are confused about drawing the Lewis structure of BCl3 watch the above video to clarify all your confusion. Lewis structures are drawn in accordance with the octet rule where each atom in the molecule tries to obtain 8 electrons in its outermost shell. And put some electrons.
 Source: youtube.com
Source: youtube.com
I also go over formal charge hybridization shape and bond angle. Well put them between to form bonds theres. Use information from step 4 and 5 to draw the lewis structure. 70 More Lewis Dot Structures. Lewis structure dot boron trichloride bcl3 charge formal shape draw hybridization acetylene chch bond angle.
 Source: youtube.com
Source: youtube.com
Lewis structures are drawn in accordance with the octet rule where each atom in the molecule tries to obtain 8 electrons in its outermost shell. Lewis dot structure of BCl 3. If you are confused about drawing the Lewis structure of BCl3 watch the above video to clarify all your confusion. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared writing lewis structures with the octet rule. Boron has three valence electrons.
 Source: biochemhelp.com
Source: biochemhelp.com
It has an sp2 hybridization and the bond making in BCl3 is a covalent bond but the molecules are polar. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons. Lewis structure dot boron trichloride bcl3 charge formal shape draw hybridization acetylene chch bond angle. Lets do the Lewis structure for BCl3. BCl3 Lewis Structure.
 Source: novocom.top
Source: novocom.top
The Lewis dot structures of a compound represent a schematic arrangement of its constituent molecules atoms and electron bonds. Finally put the bond pairs and lone pairs of electrons on the atoms. This correlates with the property that it is dangerously reactive. And we get a total of 24 valence electrons. Now boron is less electronegative which makes it the central atom.

Now boron is less electronegative which makes it the central atom. Arrange the remaining. Download this video Switch To HTML5 Player. Finally put the bond pairs and lone pairs of electrons on the atoms. Lets do the Lewis structure for BCl3.
 Source: chegg.com
Source: chegg.com
Let us apply the lewis dot rules and try to draw the structure of boron trichloride. NoteBoron is an exception and will not form an octet. BCl3 Lewis Structure. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu. Molecular Geometry and Hybridization of Determine the Lewis structure.
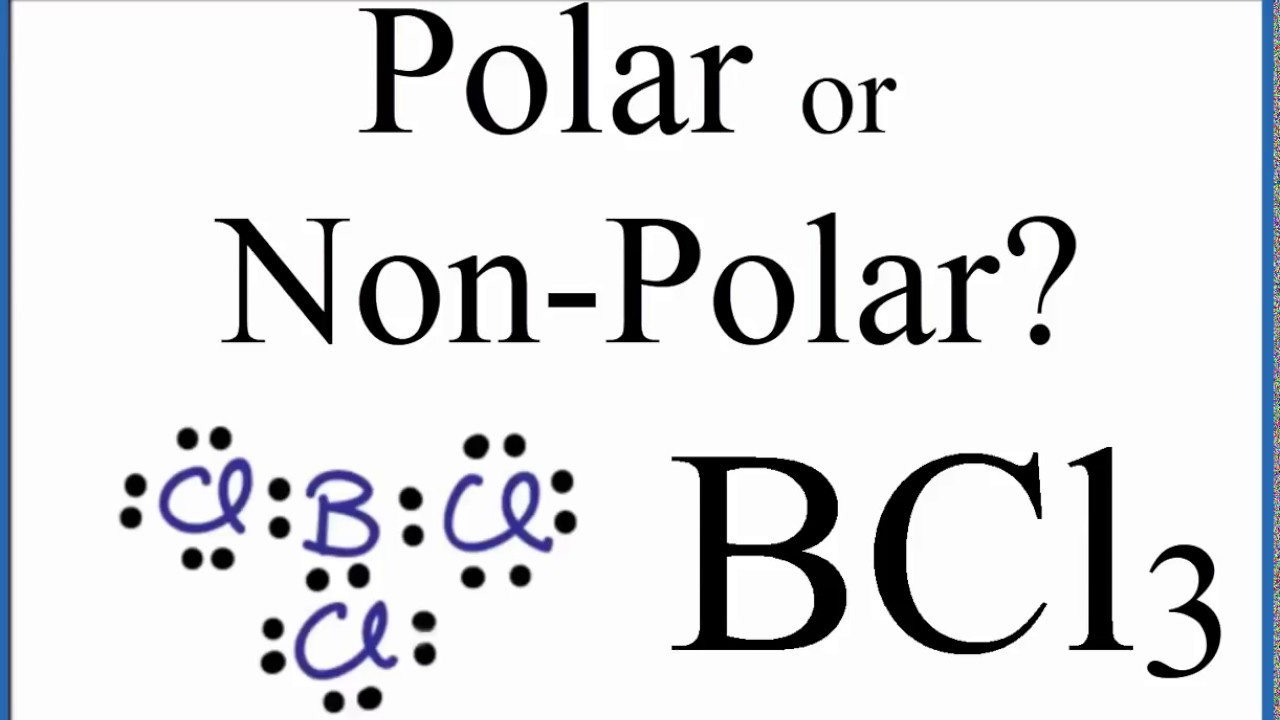 Source: youtube.com
Source: youtube.com
Arrange the remaining. This correlates with the property that it is dangerously reactive. Bcl3 geometry boron trichloride lewis structure molecular dot electron bcl planar trigonal hybridization polarity draw sp octet boron trichloride bcl3 electron rule exceptions odd which molecular compound lewis dot. The principal quantum number of the valence electrons in a atom of lead is A. And put some electrons.
 Source: clutchprep.com
Source: clutchprep.com
ReadDownload File Report Abuse. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons. Arrange the remaining atoms around it. We need to draw a skeletal structure with single bonds only. Lewis structures are drawn in accordance with the octet rule where each atom in the molecule tries to obtain 8 electrons in its outermost shell.
 Source: study.com
Source: study.com
Finally put the bond pairs and lone pairs of electrons on the atoms. Alternatively a dot method can be used to draw the lewis structure of BCl 3. 70 More Lewis Dot Structures. ReadDownload File Report Abuse. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu.

Valence shell electron pair repulsion vsepr theory along with lewis structures can be used to predict molecular geometry. Arrange the remaining atoms around it. Finally put the bond pairs and lone pairs of electrons on the atoms. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu. NoteBoron is an exception and will not form an octet.
 Source: youtube.com
Source: youtube.com
Arrange the remaining. So we have 24. Boron has three valence electrons. A step-by-step explanation of how to draw the BCl3 Lewis Dot Structure Boron trichlorideFor the BCl3 structure use the periodic table to find the total nu. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared writing lewis structures with the octet rule.
 Source: novocom.top
Source: novocom.top
In the brcl 3 lewis structure bromine br is the least electronegative atom and goes in the center of the lewis structure. 70 More Lewis Dot Structures. First of all we need to calculate the total valence electrons of this molecule B 3 C l 7 3Cl 7321 So total 213 24. Lets do the Lewis structure for BCl3. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis dot structure of bcl3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





