Lewis dot structure of clo4
Lewis Dot Structure Of Clo4. Chlorine does not follow the octet. Answered How to find the Lewis dot structure of ClO4 - 1 See answer Answers. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the. Petty11 20102019 Chemistry Secondary School 5 pts.
 Clo4 Lewis Structure Perchlorate Ion Youtube From youtube.com
Clo4 Lewis Structure Perchlorate Ion Youtube From youtube.com
The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the. In lewis structure of ClO 4-ion there are three lone pairs in the last shell in one oxygen atom and that oxygen atoms have a -1 charge on each atoms. The Questions and Answers of How can we draw Lewis structure of clo4-. Calculate the total valence electrons in the molecule. Is done on EduRev Study Group by Class 11 Students. A step-by-step explanation of how to draw the HClO4 Lewis Structure Perchloric AcidWhen we have an H or H2 in front of a polyatomic molecule like CO3.
Use information from step 4 and 5 to draw the lewis structure.
Lewis dot structure of MgF 2. A step-by-step explanation of how to draw the HClO4 Lewis Structure Perchloric AcidWhen we have an H or H2 in front of a polyatomic molecule like CO3. When you draw the Lewis structure of ClO4 1 the first structure is Cl with 4 oxygens attached by single bonds. Chlorine gives seven electrons to valence electrons. Also there is no charge in those oxygen atoms. I also go over the formal charge hybridization shape bond angle and re.
 Source: techiescientist.com
Source: techiescientist.com
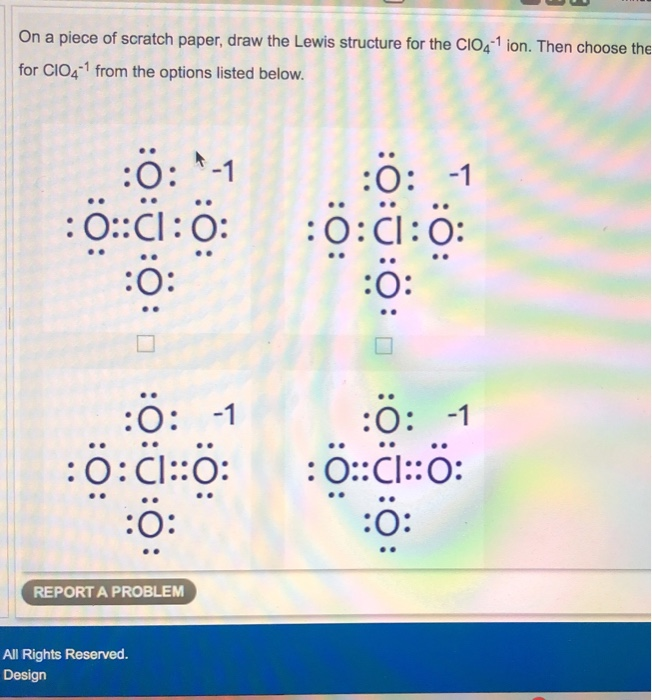
Drawing the Lewis Structure for ClO 4- Perchlorate Ion Perchlorates salts with the ClO 4- are used in rocket fuel NH 4 ClO 4 and to treat hyperthyroidism NaClO 4. The Lewis structure for ClO 4- requires you to place Chlorine Cl in the center of the structure since it is the most electronegative. Lewis dot structure of CLO 4-Alternatively a dot method can be used to draw the lewis structure. Mg has configuration 282 There are two valence electrons in Mg. Lewis structure of clo4 chemical bonding is one of the most important and interesting chapters of chemistry.
 Source: youtube.com
Source: youtube.com
Also there is no charge in those oxygen atoms. Lewis Dot Structure of ClO4- PerChlorate Ion. The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom. So the number of lone pairs of Chlorine here is 0. Remember Chlorine is in Period 3 and can hold more than 8 valence electrons.
 Source: meritnation.com
Source: meritnation.com
The ClO4-Lewis structure is a good structure to help you understand. Are solved by group of students and teacher of Class. Mg has configuration 282 There are two valence electrons in Mg. Do you know that you can find the presence of ClO4 even on Mars. Is done on EduRev Study Group by Class 11 Students.
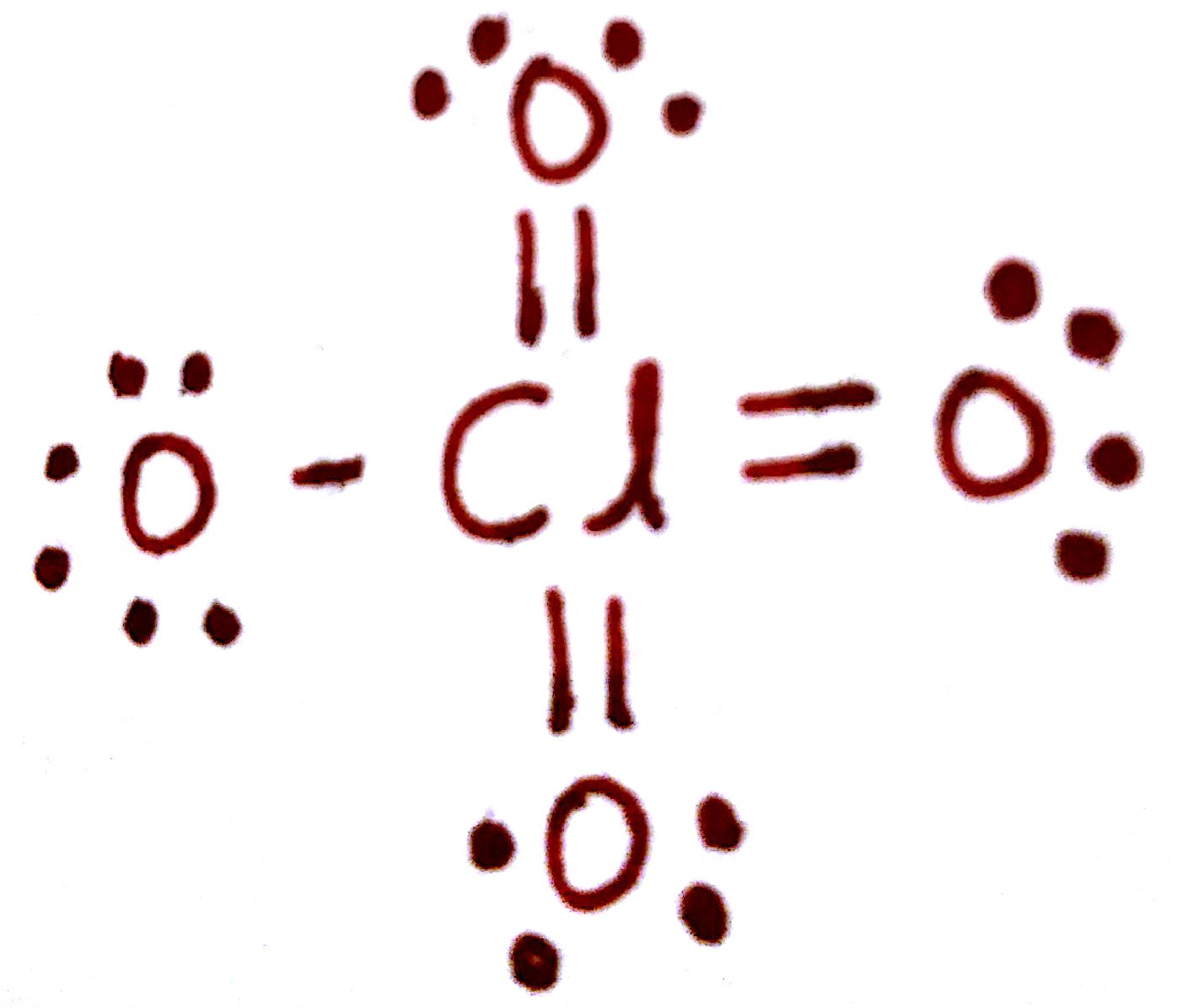 Source: novocom.top
Source: novocom.top
Lewis dot structure of MgF 2. Lewis Dot Structure of ClO4- PerChlorate Ion. The lewis structure of the carbonate ion also suggests a. How to find the Lewis dot structure of ClO4 - - 13117892 1. 1 write atom connectivity for co2.
 Source: youtube.com
Source: youtube.com
The Questions and Answers of How can we draw Lewis structure of clo4-. One example of this is a lewis dot structure. Lewis dot structure of MgF 2. A step-by-step explanation of how to draw the ClO4- Lewis Structure Perchlorate Ion. Im supposed to draw them and determine which is the most favorable.
 Source: techiescientist.com
Source: techiescientist.com
Chlorine does not follow the octet. This discussion on How can we draw Lewis structure of clo4-. The lewis structure of the carbonate ion also suggests a. Calculate the total valence electrons in the molecule. Do you know that you can find the presence of ClO4 even on Mars.
 Source: brainly.com
Source: brainly.com
Each F has one 7 valence electrons each. Lewis Dot Structure of ClO4- PerChlorate Ion. Chlorine gives seven electrons to valence electrons. In lewis structure of ClO 4-ion there are three lone pairs in the last shell in one oxygen atom and that oxygen atoms have a -1 charge on each atoms. The lewis dot structure is.
 Source: pinterest.com
Source: pinterest.com
Now for ClO4- we already have found out that chlorine is the central atom. Is done on EduRev Study Group by Class 11 Students. Mg has configuration 282 There are two valence electrons in Mg. The lewis dot structure is. I quickly take you through how to draw the Lewis Structure of ClO4- Chlorate Ion.
Source: chemspider.com
The lewis structure of the carbonate ion also suggests a. The Lewis structure for ClO 4- requires you to place Chlorine Cl in the center of the structure since it is the most electronegative. There are another three oxygen atoms. Chlorine gives seven electrons to valence electrons. I quickly take you through how to draw the Lewis Structure of ClO4- Chlorate Ion.
 Source: meritnation.com
Source: meritnation.com
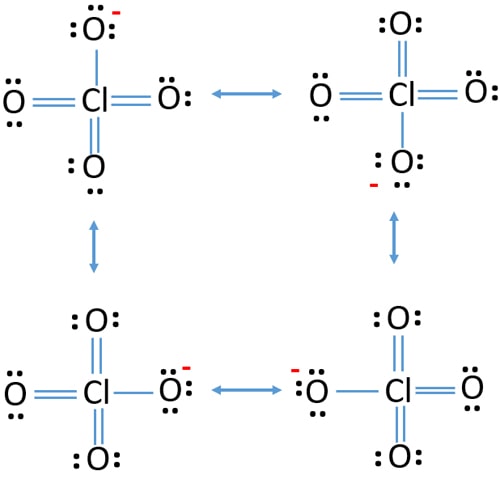
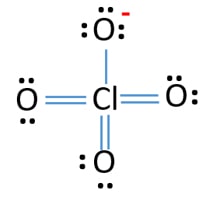
If we look closely into the lewis structure we can see that all the valence electrons around chlorine are bonded with oxygen in either single or double bonds in this case 1 single and 3 double bonds. The Lewis structure for ClO 4- requires you to place Chlorine Cl in the center of the structure since it is the most electronegative. Lewis Dot Structure of ClO4- PerChlorate Ion. The lewis dot structure is. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures.
 Source: chegg.com
Source: chegg.com
Answered How to find the Lewis dot structure of ClO4 - 1 See answer Answers. Lewis structure of clo4 lewis structure of clo4-1 lewis structure of clo4- anion. I also go over the formal charge. Calculate the total valence electrons in the molecule. Cl has 7 valence electrons Each O has six valence electrons due to the presence of negative charge on the species an excess electron is available.
 Source: chemistryscl.com
Source: chemistryscl.com
Im supposed to draw them and determine which is the most favorable. 60 Mi To Km. Chlorine does not follow the octet. Remember Chlorine is in Period 3 and can hold more than 8 valence electrons. A step-by-step explanation of how to draw the ClO4- Lewis Structure Perchlorate Ion.
 Source: biochemhelp.com
Source: biochemhelp.com
Answered How to find the Lewis dot structure of ClO4 - 1 See answer Answers. The Lewis structure for ClO 4- requires you to place Chlorine Cl in the center of the structure since it is the most electronegative. There are another three oxygen atoms. Odell Beckham 2013 Sunflower Art Van Gogh Deepest Hole On Earth Structure Of Xylulose Airsoft Custom Scar Friends Thanksgiving Football Motocross Starting Gate Wallpaper Humanstuck Nepeta People French Kissing. The Questions and Answers of How can we draw Lewis structure of clo4-.
 Source: chemistryscl.com
Source: chemistryscl.com
Those oxygen atoms are connected to the chlorine atom from double bonds and have two lone pairs in their last shell. These are some keyword suggestions for the term Lewis Structure Of Clo4. The Lewis structure for ClO 4- requires you to place Chlorine Cl in the center of the structure since it is the most electronegative. How to find the Lewis dot structure of ClO4 - - 13117892 1. Lewis Dot Structure of ClO4- PerChlorate Ion.
 Source: youtube.com
Source: youtube.com
Now for ClO4- we already have found out that chlorine is the central atom. I also go over the formal charge hybridization shape bond angle and re. Those oxygen atoms are connected to the chlorine atom from double bonds and have two lone pairs in their last shell. Also there is no charge in those oxygen atoms. Lewis Dot Structure For Clo4.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis dot structure of clo4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






