Lewis dot structure sicl4
Lewis Dot Structure Sicl4. H2S SiCl4 BeF2 HCOOH. H2S SiCl4 BeF2 CO2 - 3 and HCOOH. Silicon tetrachloride is corrosive to tissue and metal. The molecule has a grand total of 36 electrons.
It is used to produce high-quality silica for commercial purposes. Sicl4 lewis structure silicon tetrachloride draw dot diagram geometry bond molecular angle valence hybridization nonpolar polar sicl4 atoms bonding many there silicon tetrachloride sicl4 2d. For the SiCl4 Lewis structure we first count the valence electrons for the SiCl4 molecule using the periodic table. Draw the Lewis structures f. H2S SiCl4 BeF2 HCOOH. Consider a bent molecule such as H2Se in which the central atom has two lone pairs of electrons.
Draw the Lewis structures for the following molecules and ions.
1 H2S H has 1 electron in its valence shell sulphur has 6 electron its valence shell. 1 H2S H has 1 electron in its valence shell sulphur has 6 electron its valence shell. Draw Lewis Structure For Sicl4 SiCl4 Lewis Structure How to Draw the Lewis Structure Chemistry Class 11 NCERT Solutions. What is the lewis dot structure for sicl4 Add your answer and earn points. Consider a bent molecule such as H2Se in which the central atom has two lone pairs of electrons. H2S SiCl4 BeF2 HCOOH.
 Source: clutchprep.com
Source: clutchprep.com
H2S SiCl4 BeF2 CO32- HCOOH. Draw the Lewis structures f. 1 H2S H has 1 electron in its valence shell sulphur has 6 electron its valence shell. Draw Lewis Structure For Sicl4 SiCl4 Lewis Structure How to Draw the Lewis Structure Chemistry Class 11 NCERT Solutions. H2S SiCl4 BeF2 CO32- HCOOH.
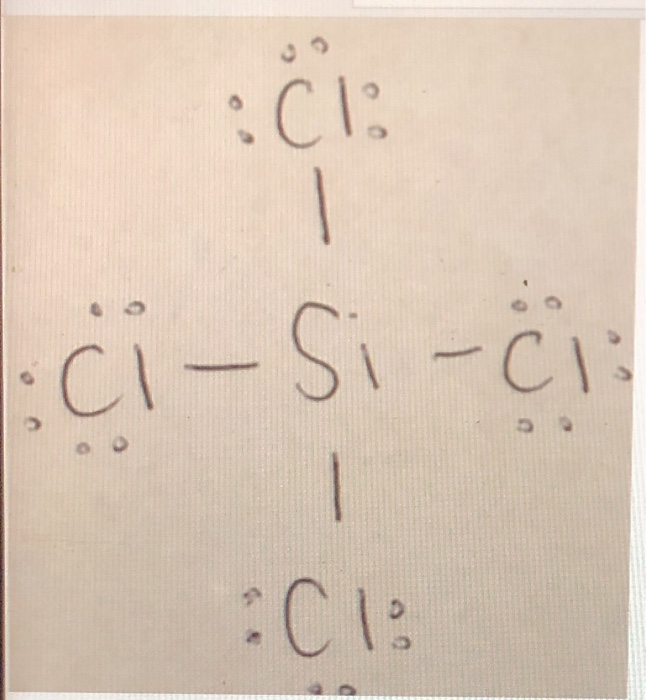 Source: chegg.com
Source: chegg.com
Draw Lewis Structure For Sicl4 SiCl4 Lewis Structure How to Draw the Lewis Structure Chemistry Class 11 NCERT Solutions. In this article we will discuss Silicon tetrachloride SiCl4 lewis dot structure molecular geometry hybridization polar or nonpolar etc. February 19 2021. It is used to produce high-quality silica for commercial purposes. How to Draw the Lewis Structure for SiH4 Lewis Structure Molecular Geometry YouTube Silicon Tetrafluoride SiF4 Lewis Dot Structure YouTube.
 Source: clutchprep.com
Source: clutchprep.com
A planar structure will be formed. What is the Lewis dot structure of SiCl4. Draw the Lewis structures f. For the SiCl4 Lewis structure we first count the valence electrons for the SiCl4 molecule using the periodic table. The molecule consists of a central Si atom which has a coordination.
![]() Source: commons.wikimedia.org
Source: commons.wikimedia.org
High purity of silicon tetrachloride used in the manufacture of optical fibers. Silicon tetrachloride is corrosive to tissue and metal. The molecule has a grand total of 36 electrons. What is the lewis dot structure for sicl4 Add your answer and earn points. Lewis Dot Structure For Sicl4 SiCl4 Lewis Structure How to Draw the Lewis Structure SCl4 Lewis Structure.
 Source: novocom.top
Source: novocom.top
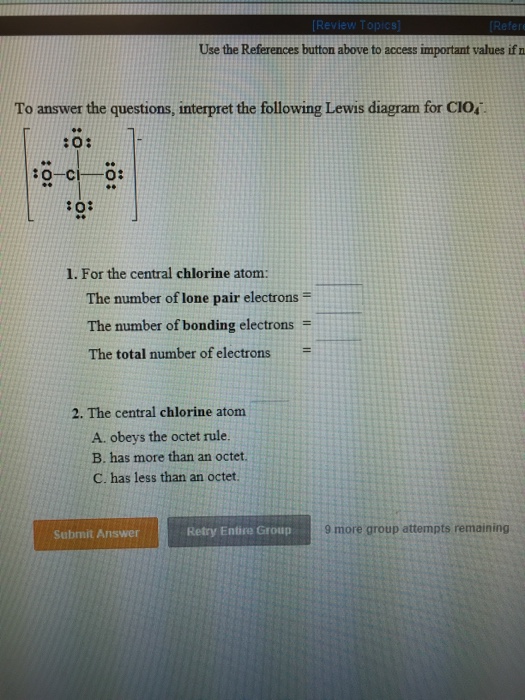
Draw the Lewis structures for the following molecules and ions. 2 Si has 4 electrons its valence shell Cl need only 1 electron to complete its octet therefore 4 Cl will make 4 bonds with SiA tetrahedral shape will be formed. The central atom has no lone pair and there are four bond pairs. Silicon Si is the least electronegative atom and goes at the center of the structure. Basics of Chemical Bonding.
 Source: youtube.com
Source: youtube.com
The Lewis structure of a compound provides the arrangement of the valence electrons of the compound. Draw Lewis Structure For Sicl4 SiCl4 Lewis Structure How to Draw the Lewis Structure Chemistry Class 11 NCERT Solutions. How to Draw the Lewis Structure for SiH4 Lewis Structure Molecular Geometry YouTube Silicon Tetrafluoride SiF4 Lewis Dot Structure YouTube. The molecule has a grand total of 36 electrons. Lewis Dot Diagram Structure For Sicl4 Molecular Geometry Bond Angle Hybridization Polar Nonpolar Bohr Rutherford Diagrams Amp Lewis Dot Diagrams Eve Wongworakul Bohr Rutherford Diagrams Amp Lewis Dot Diagrams Eve Wongworakul What Is The Electron Dot Diagram For The Element Silicon Ppt Lewis Electron Dot Diagrams Notation Orbital And Lewis Dot Shmoop Bagikan.
 Source: novocom.top
Source: novocom.top
These electrons are drawn as lines for bonding electrons and pairs of dots for nonbonding. Basics of Chemical Bonding. The molecule consists of a central Si atom which has a coordination. These electrons are drawn as lines for bonding electrons and pairs of dots for nonbonding. Draw the Lewis structures for the following molecules and ions.
 Source: novocom.top
Source: novocom.top
A planar structure will be formed. The central atom has no lone pair and there are four bond pairs. Draw the Lewis structures f. High purity of silicon tetrachloride used in the manufacture of optical fibers. What is the Lewis dot structure of SiCl4.
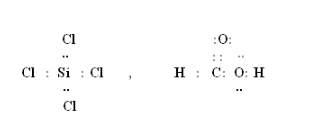 Source: ask.learncbse.in
Source: ask.learncbse.in
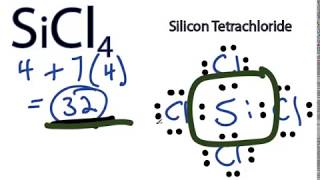
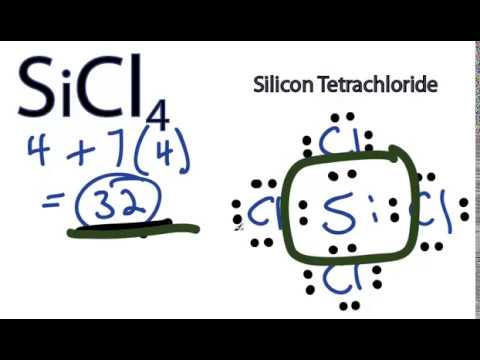
SiCl 4 is similar to the SiF 4 Lewis structure. A step-by-step explanation of how to draw the SiCl4 Lewis Dot Structure Silicon tetrachlorideFor the SiCl4 structure use the periodic table to find the to. A step-by-step explanation of how to draw the AlCl4- Lewis Dot StructureFor the AlCl4- structure use the periodic table to find the total number of valence. SiCl4 Lewis Structure - How to Draw the Lewis Structure for SiCl4. What is the lewis dot structure for sicl4 Add your answer and earn points.
 Source: youtube.com
Source: youtube.com
In this article we will discuss Silicon tetrachloride SiCl4 lewis dot structure molecular geometry hybridization polar or nonpolar etc. A step-by-step explanation of how to draw the SiCl4 Lewis Dot Structure Silicon tetrachlorideFor the SiCl4 structure use the periodic table to find the to. Sicl4 lewis structure silicon tetrachloride draw dot diagram geometry bond molecular angle valence hybridization nonpolar polar sicl4 atoms bonding many there silicon tetrachloride sicl4 2d. 2 Si has 4 electrons its valence shell Cl need only 1 electron to complete its octet therefore 4 Cl will make 4 bonds with SiA tetrahedral shape will be formed. TetrahedralSo SiCl4 has a tetrahedral shape.
 Source: clutchprep.com
Source: clutchprep.com
Draw the Lewis structures for the following molecules and ions. SiCl 4 is similar to the SiF 4 Lewis structure. The molecule consists of a central Si atom which has a coordination. Consider a bent molecule such as H2Se in which the central atom has two lone pairs of electrons. Silicon Si is the least electronegative atom and goes at the center of the structure.
 Source: youtube.com
Source: youtube.com
H2S SiCl4 BeF2 CO32- HCOOH. Quiz your students on Lewis Dot Diagram Structure For SiCl4 Molecular Geometry Bond Angle Hybridization PolarNonpolar using our fun classroom quiz. How to Draw the Lewis Structure for SiH4 Lewis Structure Molecular Geometry YouTube Silicon Tetrafluoride SiF4 Lewis Dot Structure YouTube. How to Draw the Lewis Structure for Draw the Lewis structures for the following molecules and. Draw the Lewis structures for the following molecules and ions.

In this article we will discuss Silicon tetrachloride SiCl4 lewis dot structure molecular geometry hybridization polar or nonpolar etc. How to Draw the Lewis Structure for SiH4 Lewis Structure Molecular Geometry YouTube Silicon Tetrafluoride SiF4 Lewis Dot Structure YouTube. 1 H2S H has 1 electron in its valence shell sulphur has 6 electron its valence shell. 2 Si has 4 electrons its valence shell Cl need only 1 electron to complete its octet therefore 4 Cl will make 4 bonds with SiA tetrahedral shape will be formed. Draw the Lewis structures f.
 Source: quora.com
Source: quora.com
These electrons are drawn as lines for bonding electrons and pairs of dots for nonbonding. TetrahedralSo SiCl4 has a tetrahedral shape. Silicon tetrachloride is corrosive to tissue and metal. H2S SiCl4 BeF2 CO2 - 3 and HCOOH. What is the shape of SiCl4.
 Source: quizlet.com
Source: quizlet.com
The molecule has a grand total of 36 electrons. What is the shape of SiCl4. High purity of silicon tetrachloride used in the manufacture of optical fibers. Sicl4 lewis structure silicon tetrachloride draw dot diagram geometry bond molecular angle valence hybridization nonpolar polar sicl4 atoms bonding many there silicon tetrachloride sicl4 2d. H2S SiCl4 BeF2 HCOOH.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis dot structure sicl4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





