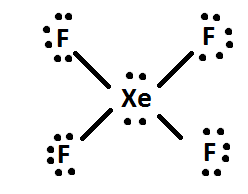
Lewis dot structure xef4
Lewis Dot Structure Xef4. Identify each first as being a simple ion polyatomic ion ionic compound with or without a. Find octet e- for each atom and add them together. It is a useful concept to understand and analyze the reactivity polarity color phase of matter magnetism and so on. I also go over hybridization shape and bond angle.
 How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube From youtube.com
How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube From youtube.com
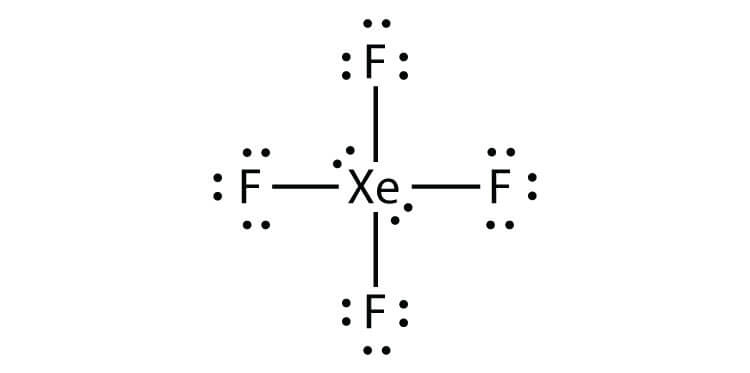
For the SO3 2- Lewis structure the total number of valence electrons. A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. Drawing XeF4 Lewis Structure is very easy to by using the following method. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. XeF4 Lewis Structure Now that we know the valence electrons of Xenon Tetrafluoride it will be easier for you to draw its Lewis structure. Each fluorine atom has three lone pairs.
Let us find out the lewis structure of xenon tetrafluoride.
Molecular Geometry of XeF4 The geometry of molecules which is also commonly known as molecular structure is a 3-D structure of the entire molecule. Does bohr disapprove of rutherfords idea on the atoms structure. Find valence e- for all atoms. How_to_draw_the_lewis_dot_structure_for_xef4 24 How To Draw The Lewis Dot Structure For Xef4 Download How To Draw The Lewis Dot Structure For Xef4 how to draw the lewis A step-by-step explanation of how to draw the SO3 2- Lewis Structure Sulfite Ion. Chemistry tutorial for the lewis dot structure. First draw the lewis dot structure.
 Source: biochemhelp.com
Source: biochemhelp.com
Access your email find thousands of high-quality videos and get the latest news and information. Each fluorine atom has three lone pairs. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. So its a nice tool to explore how atoms bond into. They are simply attracted to it.
 Source: novocom.top
Source: novocom.top
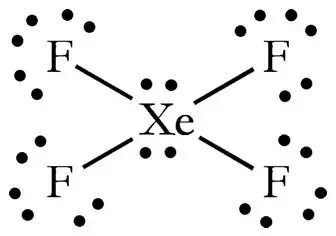
In XeF4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs around xenon atom. ABC Action News WestNet-HD the home for WestNet Wireless High-Speed Internet customers in Calgary Alberta Santa Barbara California. For the SO3 2- Lewis structure the total number of valence electrons. So we have an mx4e2 structure which is according to vsepr theory an octahedral electronic geometry means it has square planar molecular geometry. I quickly take you through how to draw the Lewis Structure of XeF4 Xenon TetraFluoride.
 Source: study.com
Source: study.com
In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. Step method to draw lewis structure for XeF4 This molecules is an example of expanded octet Step 1. As there are fluorine molecules on both the side of the central atom there is no dipole moment and hence there is no polarity. Molecular Geometry of XeF4 The geometry of molecules which is also commonly known as molecular structure is a 3-D structure of the entire molecule.
 Source: techiescientist.com
Source: techiescientist.com
Molecular Geometry of XeF4 The geometry of molecules which is also commonly known as molecular structure is a 3-D structure of the entire molecule. In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. Lewis and Three-Dimensional Structures. We also need to check to make sure we only used. Xenon has 8 dots 4 pairs of dots around the letters Xe.
 Source: youtube.com
Source: youtube.com
In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. Find octet e- for each atom and add them together. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. Here in this post we described step by step method to construct XeF4 Lewis. In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure.
 Source: youtube.com
Source: youtube.com
The Lewis structure theory does not comprehend the shape of a molecule. Step method to draw lewis structure for XeF4 This molecules is an example of expanded octet Step 1. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure.
 Source: socratic.org
Source: socratic.org
I also go over hybridization shape and bond angle. Does bohr disapprove of rutherfords idea on the atoms structure. ABC Action News WestNet-HD the home for WestNet Wireless High-Speed Internet customers in Calgary Alberta Santa Barbara California. We also need to check to make sure we only used. How_to_draw_the_lewis_dot_structure_for_xef4 24 How To Draw The Lewis Dot Structure For Xef4 Download How To Draw The Lewis Dot Structure For Xef4 how to draw the lewis A step-by-step explanation of how to draw the SO3 2- Lewis Structure Sulfite Ion.
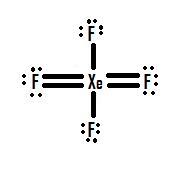 Source: chem.libretexts.org
Source: chem.libretexts.org
XeF4 Lewis Structure Now that we know the valence electrons of Xenon Tetrafluoride it will be easier for you to draw its Lewis structure. Step-by-step tutorial for drawing the Lewis Structure for XeF4. So its a nice tool to explore how atoms bond into. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. Access your email find thousands of high-quality videos and get the latest news and information.
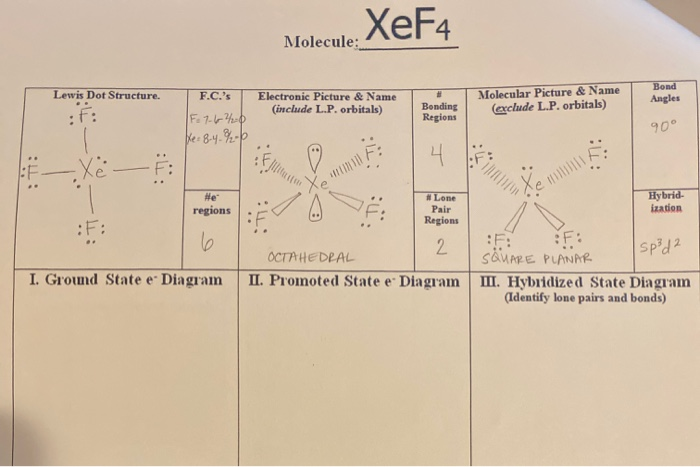 Source: chegg.com
Source: chegg.com
For the SO3 2- Lewis structure the total number of valence electrons. So its a nice tool to explore how atoms bond into. XeF4 Lewis Structure Now that we know the valence electrons of Xenon Tetrafluoride it will be easier for you to draw its Lewis structure. Lewis Dot Structure For Xef4. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less.
 Source: people.uwplatt.edu
Source: people.uwplatt.edu
The Lewis structure for XeF4 has a total of 36 valence electrons. Here in this post we described step by step method to construct XeF4 Lewis. The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe. Lewis and Three-Dimensional Structures. Therefore this molecule is nonpolar.
 Source: quora.com
Source: quora.com
In this tutorial we will learn how to draw lewis structure of XeF4 step by step. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. I also go over hybridization shape and bond angle. Does bohr disapprove of rutherfords idea on the atoms structure. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
 Source: techiescientist.com
Source: techiescientist.com
So we have an mx4e2 structure which is according to vsepr theory an octahedral electronic geometry means it has square planar molecular geometry. Hydrogen H only needs two valence electrons to have a full outer shell. How_to_draw_the_lewis_dot_structure_for_xef4 24 How To Draw The Lewis Dot Structure For Xef4 Download How To Draw The Lewis Dot Structure For Xef4 how to draw the lewis A step-by-step explanation of how to draw the SO3 2- Lewis Structure Sulfite Ion. This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. I quickly take you through how to draw the Lewis Structure of XeF4 Xenon TetraFluoride.
 Source: youtube.com
Source: youtube.com
Each fluorine atom has three lone pairs. How_to_draw_the_lewis_dot_structure_for_xef4 24 How To Draw The Lewis Dot Structure For Xef4 Download How To Draw The Lewis Dot Structure For Xef4 how to draw the lewis A step-by-step explanation of how to draw the SO3 2- Lewis Structure Sulfite Ion. I quickly take you through how to draw the Lewis Structure of XeF4 Xenon TetraFluoride. Hydrogen H only needs two valence electrons to have a full outer shell. In this tutorial we will learn how to draw lewis structure of XeF4 step by step.
 Source: socratic.org
Source: socratic.org
We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. Therefore this molecule is nonpolar. Find valence e- for all atoms. The Lewis structure theory does not comprehend the shape of a molecule. A video explanation of how to draw the Lewis Dot Structure for Xenon Tetrachloride along with information about the compound including Formal Charges Polar.
 Source: youtube.com
Source: youtube.com
Access your email find thousands of high-quality videos and get the latest news and information. In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. First draw the lewis dot structure. Xenon Xe can have more than 8 valence electrons in your Lewis structure. Find octet e- for each atom and add them together.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis dot structure xef4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.