Lewis structure for bf4
Lewis Structure For Bf4. Other questions on the subject. The BF 4-Lewis structure has a total of 32 valence electrons. What is the Lewis structure of BF4. The molecular geometry shape of BF4- is tetrahedral.
 Using The Lewis Structure Predict The Geometrical Chegg Com From chegg.com
Using The Lewis Structure Predict The Geometrical Chegg Com From chegg.com
Know how to determine the valence electron for all elements. It is a conjugate base of a tetrafluoroboric acid. They are all decomposition reactions. Made with Explain Everything. The BF 4-Lewis structure has a total of 32 valence electrons. Chemistry 21062019 2000 pal23.
A step-by-step explanation of how to draw the BH4- Lewis Dot Structure Tetrahydroborate IonFor the BH4- structure use the periodic table to find the total.
For the BH4- Lewis structure calculate the total number of val. For each give i the. Solved Draw A Lewis Structure For Each Of The Following M. 3 47 1 32. Filesilver Tetrafluoroboratepng Wikimedia Commons. Please note none of the solutions are using the expanded octet rule or formal charges O 2.
 Source: chegg.com
Source: chegg.com
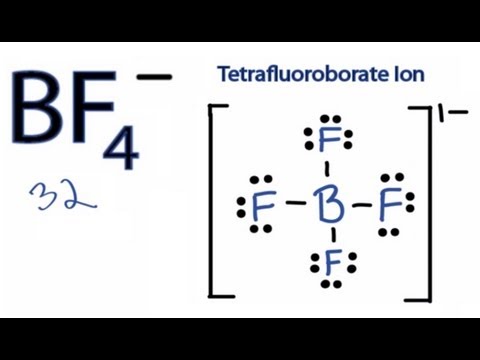
Other questions on the subject. Tetrafluoroborate is under investigation in clinical trial NCT02907073 Positron Emission Tomography PET Imaging Studies With NIS Reporter. A step-by-step explanation of how to draw the BF4- Lewis Structure. Tetrafluoroborate 1- is a boron fluoride. The Science Of Studying Lewis Dot Structure For Bf4.
 Source: youtube.com
Source: youtube.com
A step-by-step explanation of how to draw the BF3 Lewis Dot Structure Boron TrifluorideFor the BF3 Lewis structure calculate the total number of valence. Write Lewis structures for the following. Boron has 3 valence electrons and each of the four fluorides contributes one electron to each covalent bond. Know how to determine the valence electron for all elements. It is a conjugate base of a tetrafluoroboric acid.
 Source: youtube.com
Source: youtube.com
F prefers to forms only one 1 bond when not central atom. This reaction may be written in either of. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA. Solved Draw A Lewis Structure For Each Of The Following M. Please note none of the solutions are using the expanded octet rule or formal charges O 2.
 Source: youtube.com
Source: youtube.com
Note that the central atom in the Lewis structure of moleculeion is the one which has the least electronegativity. The BF 4-Lewis structure has a total of 32 valence electrons. Now the Lewis structure of BF 4 is. It is a conjugate base of a tetrafluoroboric acid. Bh4 Lewis Structure How To Draw The Lewis Structure For.
 Source: youtube.com
Source: youtube.com
Other questions on the subject. The BF 4-Lewis structure has a total of 32 valence electrons. Note that the central atom in the Lewis structure of moleculeion is the one which has the least electronegativity. Chemistry 21062019 2000 pal23. They are all decomposition reactions.
 Source: clutchprep.com
Source: clutchprep.com
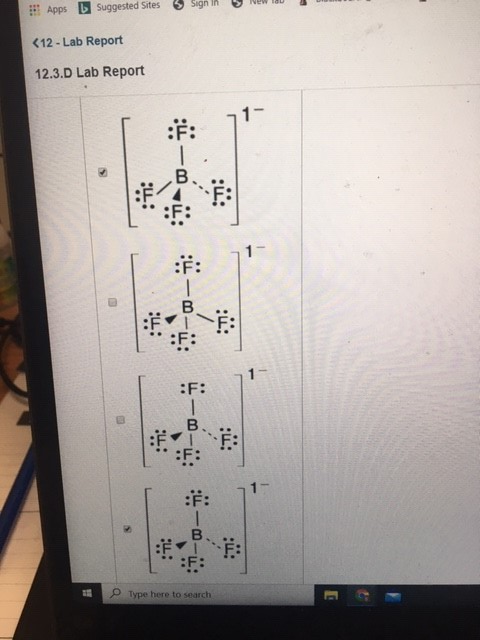
It is a conjugate base of a tetrafluoroboric acid. For the BH4- Lewis structure calculate the total number of val. 3 47 1 32. You need to put brackets around the BF 4 - Lewis structure as well as a negative charge to show that the structure is a negative ion. Lewis Dot Structure For Bf4.
 Source: answer-me-up.com
Source: answer-me-up.com
For the BH4- Lewis structure calculate the total number of val. Filesilver Tetrafluoroboratepng Wikimedia Commons. They are all decomposition reactions. Bf4- lewis structure Phrases contain exact bf4- lewis structure from credible sources. Solved Draw A Lewis Structure For Each Of The Following M.

This reaction may be written in either of. Filenitrosonium Tetrafluoroborate 2dpng Wikimedia Commons. Bh4 Lewis Structure How To Draw The Lewis Structure For. Write Lewis structures for the following. Solved E 20 What Is.
 Source: study.com
Source: study.com
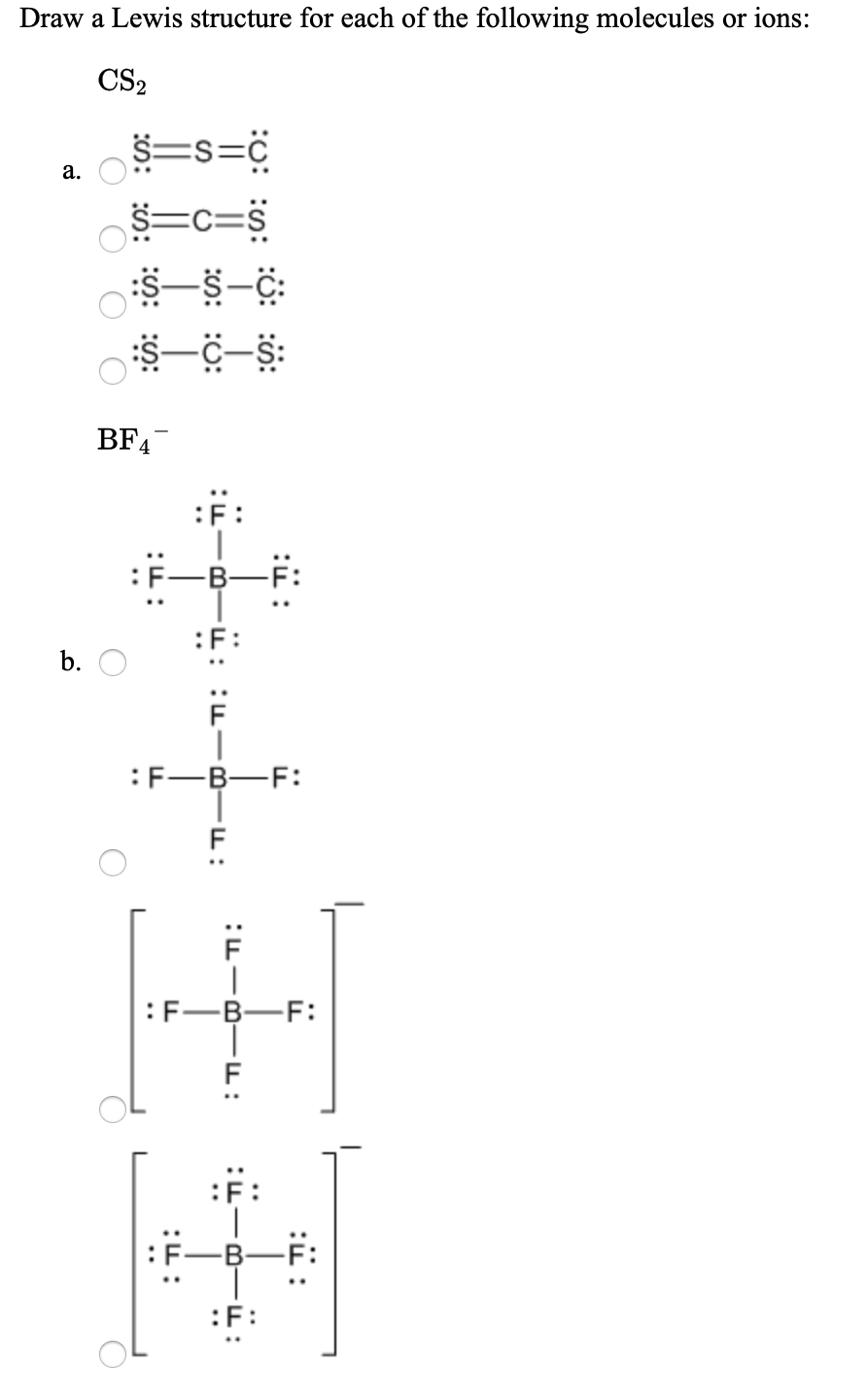
Solved E 20 What Is. Please note none of the solutions are using the expanded octet rule or formal charges O 2. Can u anwser my question Ill pay you 20 if its right I have cashapp PayPal. ClCN ceC22 Answer a. A step-by-step explanation of how to draw the BF4- Lewis Structure.
 Source: chegg.com
Source: chegg.com
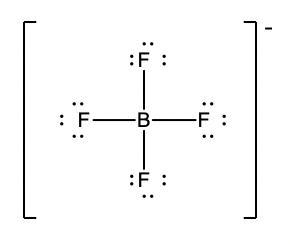
Lewis dot structure includes the atoms involved in the molecule and how they get bonded using their valence electrons. Write the Lewis structure for BF4- How many single bonds are there. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA. Lewis dot structure includes the atoms involved in the molecule and how they get bonded using their valence electrons. Bf4 lewis structureNow the Lewis structure of BF 4 BF 4 is.
 Source: quora.com
Source: quora.com
Bf4- lewis structure Phrases contain exact bf4- lewis structure from credible sources. Solved E 20 What Is. The BF 4-Lewis structure has a total of 32 valence electrons. The molecular geometry shape of BF4- is tetrahedral. Lewis dot structure includes the atoms involved in the molecule and how they get bonded using their valence electrons.
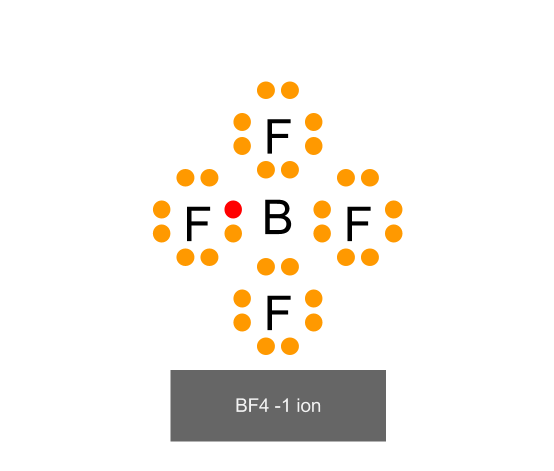 Source: scienceofstudying.blogspot.com
Source: scienceofstudying.blogspot.com
H 3 O ceNH4 ceBF4- HCCH. Lewis structure is very important in chemistry because they are used in many important concepts of general chemistry such as chemical bonding resonance valence shell we can learn to make accurate lewis dot structures in 4 simple steps. It is a conjugate base of a tetrafluoroboric acid. So the structure is shown below- Boron caries the negative charge and possess. Filesilver Tetrafluoroboratepng Wikimedia Commons.

Bh4 Lewis Structure How To Draw The Lewis Structure For. Lewis Dot Structure For Bf4. It derives from a hydride of a borohydride. This reaction may be written in either of. For the BH4- Lewis structure calculate the total number of val.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
Tetrafluoroborate 1- is a boron fluoride. Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA. Tetrafluoroborate 1- is a boron fluoride. 3 47 1 32. Agas in a balloon at.
 Source: youtube.com
Source: youtube.com
Tetrafluoroborate 1- is a boron fluoride. Lewis structure is very important in chemistry because they are used in many important concepts of general chemistry such as chemical bonding resonance valence shell we can learn to make accurate lewis dot structures in 4 simple steps. The molecular geometry shape of BF4- is tetrahedral. 3 47 1 32. Boron has 3 valence electrons and each of the four fluorides contributes one electron to each covalent bond.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis structure for bf4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





