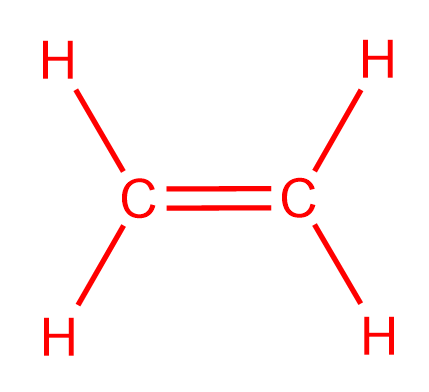
Lewis structure for c2h4
Lewis Structure For C2h4. Water is a watter that can be drabk and can be differnt types of water ex. However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. Lewis structure for c2h4. Valence and the Structure of Atoms and Molecules-Gilbert Newton Lewis 1923 Chemical Principles for Organic Chemistry-Robert Boikess 2014-01-01 Covering all the concepts that carry over from general chemistry to the organic course CHEMICAL PRINCIPLES FOR ORGANIC CHEMISTRY helps you unlearn some of the approaches you learned in General Chemistry learn new or different ones and.
 Lewis Structure Of C2h4 Biochemhelp From biochemhelp.com
Lewis Structure Of C2h4 Biochemhelp From biochemhelp.com
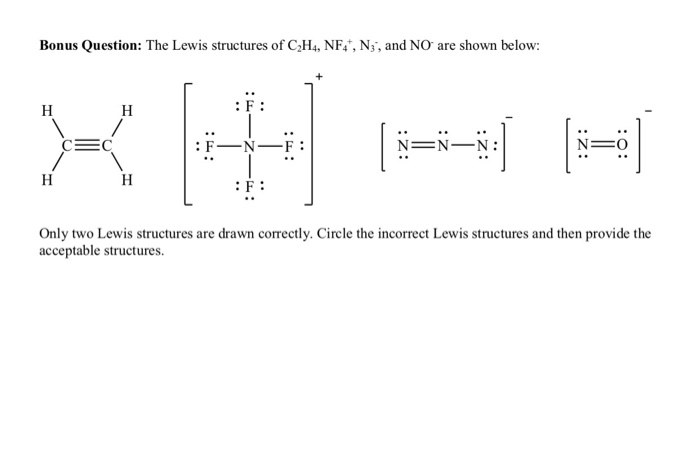
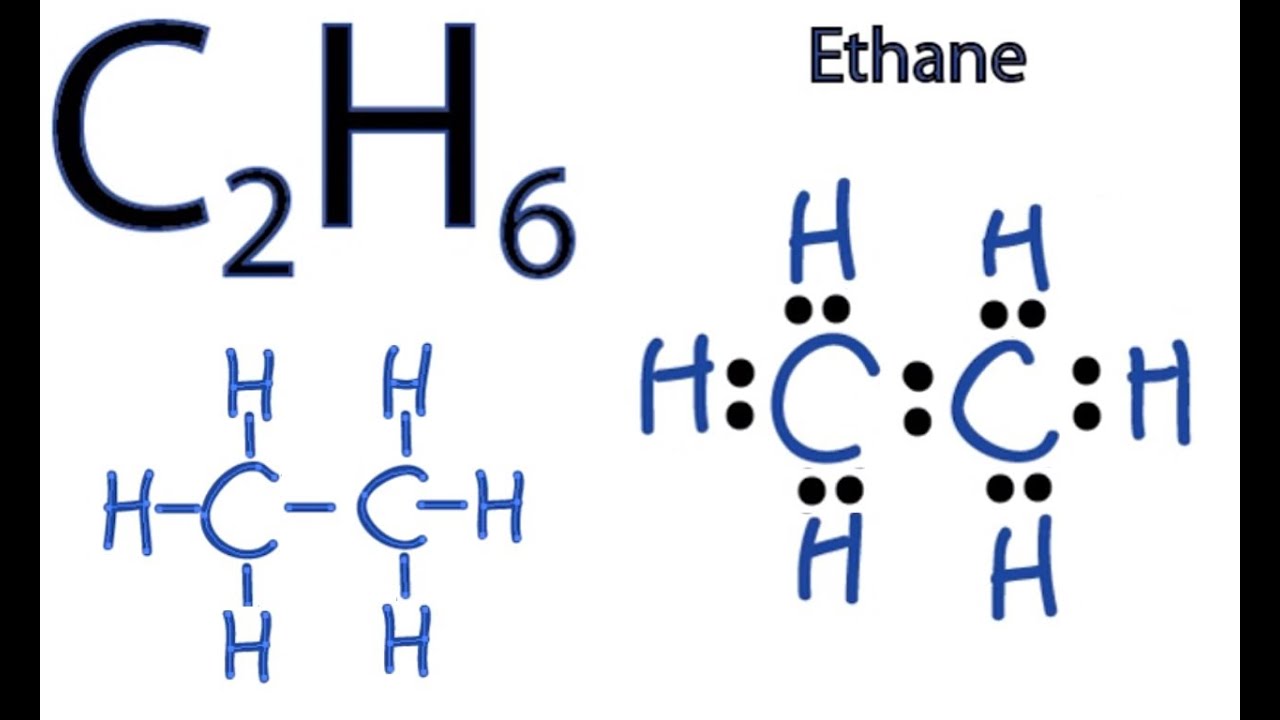
I H H H CC 7 I H O H H-C-C I-0-1. No lone pair is present on the central or outer atom in the lewis structure of ethene. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. We place two valence electrons between each atom as shown in the figure. Start by forming covalent bonds between the Carbon and Hydrogen atoms.
Therefore there cannot be more than one stable resonance structure for C.
PSidk what else to say. Start by forming covalent bonds between the Carbon and Hydrogen atoms. Electron Dot Structure for ethane C2H4. This is due to the fact that each carbon surrounds a planar triangle. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. Lewis dot structure of C 2 H 4.
 Source: chegg.com
Source: chegg.com
Experts are tested by Chegg as specialists in their subject area. Water is a watter that can be drabk and can be differnt types of water ex. Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr. Watch the video of Dr. There are two triangles overlapping each other as we can see in the diagram.
 Source: novocom.top
Source: novocom.top
However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. Who are the experts. No lone pair is present on the central or outer atom in the lewis structure of ethene. This is due to the fact that each carbon surrounds a planar triangle. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair.
 Source: clutchprep.com
Source: clutchprep.com
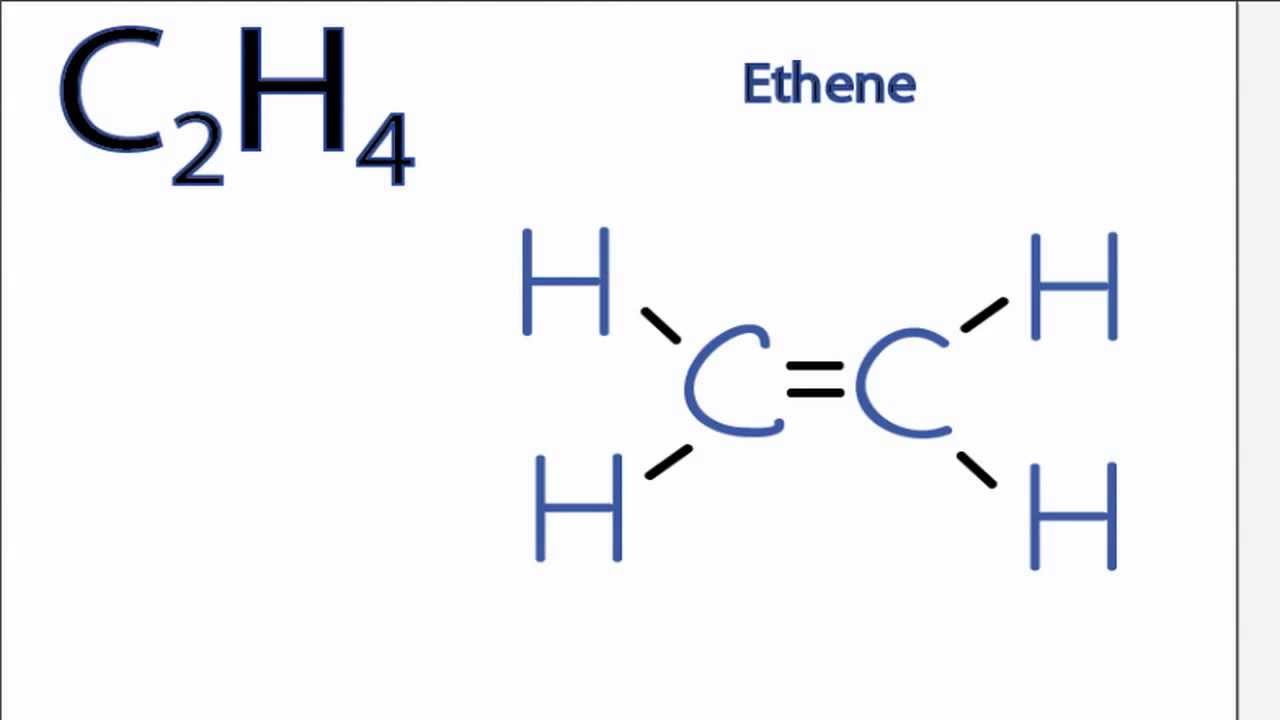
Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr. To draw the Lewis structure for C2H4 the total number of valence electrons must be known. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Lewis structure for c2h4. No lone pair is present on the central or outer atom in the lewis structure of ethene.
 Source: youtube.com
Source: youtube.com
There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell. Lewis structure for c2h4. We review their content and use your feedback. C2H4 Lewis StructureHow do you draw the Lewis structure for C2H4DescriptionTo draw the C2H4 Lewis structureyou need to find out the valence electrons in. Identify the correct Lewis structure for C2H4.
 Source: topblogtenz.com
Source: topblogtenz.com
Therefore there cannot be more than one stable resonance structure for C. C2H4 Lewis Structure. A Draw The Lewis Structure For Ethylene C2H4. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. These are covalent bonds.
 Source: youtube.com
Source: youtube.com
C2h4 Lewis Structure Page 2 Line 17qq Com. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. We review their content and use your feedback. C2h4 Lewis Structure Worksheet Printable Worksheets and. See the answer See the answer See the answer done loading.
 Source: clutchprep.com
Source: clutchprep.com
Therefore there cannot be more than one stable resonance structure for C. Water is a watter that can be drabk and can be differnt types of water ex. C2h4 Lewis Structure Page 2 Line 17qq Com. No lone pair is present on the central or outer atom in the lewis structure of ethene. I H H H CC 7 I H O H H-C-C I-0-1.
 Source: chemistryscl.com
Source: chemistryscl.com
Electron Dot Structure for ethane C2H4. For c2h4you have a total of 12 total valence electrons. Draw the electron dot structure of ethene C2H4. C-C I H O H H C-C. See the answer See the answer See the answer done loading.
 Source: brainly.in
Source: brainly.in
Lewis structure of C2H4. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them. C-C I H O H H C-C. C2H4 Lewis StructureHow do you draw the Lewis structure for C2H4DescriptionTo draw the C2H4 Lewis structureyou need to find out the valence electrons in. DIAGRAM Dot Diagram Of C2h4 FULL Version HD Quality Of C2h4.
![]() Source: study.com
Source: study.com
Watch the video of Dr. These are covalent bonds. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells. There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell. Water is a watter that can be drabk and can be differnt types of water ex.
 Source: chemistryscl.com
Source: chemistryscl.com
Covid 19 is an emerging rapidly evolving situation. We place two valence electrons between each atom as shown in the figure. Water is a watter that can be drabk and can be differnt types of water ex. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Lewis structure for c2h4.
 Source: biochemhelp.com
Source: biochemhelp.com
Answer. PSidk what else to say. Alternatively a dot method can be used to draw the lewis structure. Water in lakessalty watter while oil is a substance that is slippery and bad smell and taste and it is malansa if you know whats malansa means. The Lewis structure of C2 H4 also known as ethene has two carbons with a double bond between them.
 Source: youtube.com
Source: youtube.com
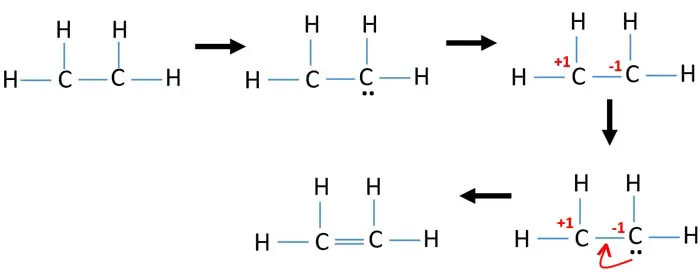
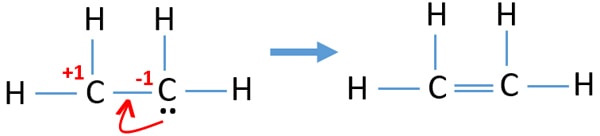
Therefore there cannot be more than one stable resonance structure for C. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. This means that the carbon atoms share 4 electrons. Draw the electron dot structure of ethene C2H4. We review their content and use your feedback.
 Source: techiescientist.com
Source: techiescientist.com
PSidk what else to say. Water is a watter that can be drabk and can be differnt types of water ex. I H H H CC 7 I H O H H-C-C I-0-1. Hydrogen is the least electronegative element here. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
 Source: techiescientist.com
Source: techiescientist.com
C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. Watch the video of Dr. DIAGRAM Dot Diagram Of C2h4 FULL Version HD Quality Of C2h4. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for c2h4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.