Lewis structure for ch3cn
Lewis Structure For Ch3cn. We can take 2 valence electrons from the Nitrogen here and move it to the center to form a double bond. 1 x 5 5 each oxygen has six valence electrons. Posted on 13122020 13122020. Heres what I got.
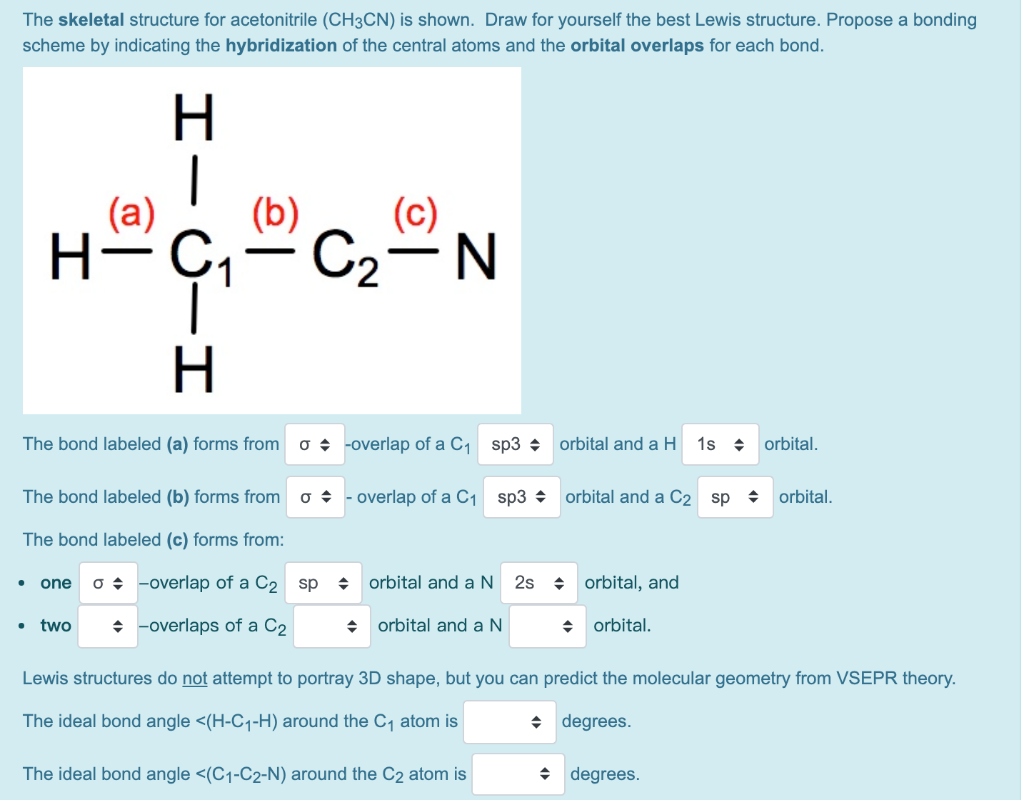 The Skeletal Structure For Acetonitrile Ch3cn Is Chegg Com From chegg.com
The Skeletal Structure For Acetonitrile Ch3cn Is Chegg Com From chegg.com
Acetonitrile is the chemical compound with the. Carbon C is the least electronegative atom and goes at the center of the CH 3 CN Lewis structure. 1 x 6 6 total valence electrons. There are several lewis structures for a nitrile oxide. According to VSEPR molecules will have a geometric shape that allows their negative charge centers bonded and lone pair electrons to be as far away from each other as possible due to the mutual repulsion of the negative charges. Your answer choice is independent of the orientation of your drawn structure.
HCL interferes with the regular use of Oxygen by the organs of the body.
SpaceChem - acetonitrile benzole CH3CN - C6H6 PhH 4 years ago. Ch3oh is lewis base because carbon has completely filled orbital by sharing electron with oxygen. Your answer choice is independent of the orientation of your drawn structure. Molecules can form when atoms bond together by sharing electrons and can be represented by a useful shorthand called lewis structures. Use resonance structures to account for the acidity of acetonitrile. The molecule is bent.
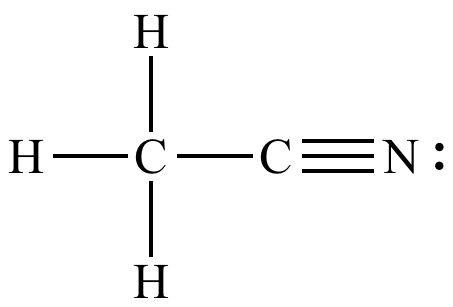 Source: chem.ucla.edu
Source: chem.ucla.edu
A step-by-step explanation of how to draw the CH3CN Lewis Dot Structure Acetonitrile For the CH3CN structure use the periodic table to find the total numb. Remember Hydrogen only needs 2 for a full outer shell. There is one lone pair on the nitrogen atom and a total of 7 bonded pairs present in the CH3CN lewis structure. The exposure can be rapidly fatal. According to VSEPR molecules will have a geometric shape that allows their negative charge centers bonded and lone pair electrons to be as far away from each other as possible due to the mutual repulsion of the negative charges.
 Source: youtube.com
Source: youtube.com
For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons. Lets do the CH3CN Lewis structure. A step-by-step explanation of how to draw the CH3CN Lewis Dot Structure Acetonitrile For the CH3CN structure use the periodic table to find the total numb. Understanding the molecular structure of a compound can help determine the polarity reactivity phase of matter color magnetism as well as the biological activity. Posted on 13122020 13122020.
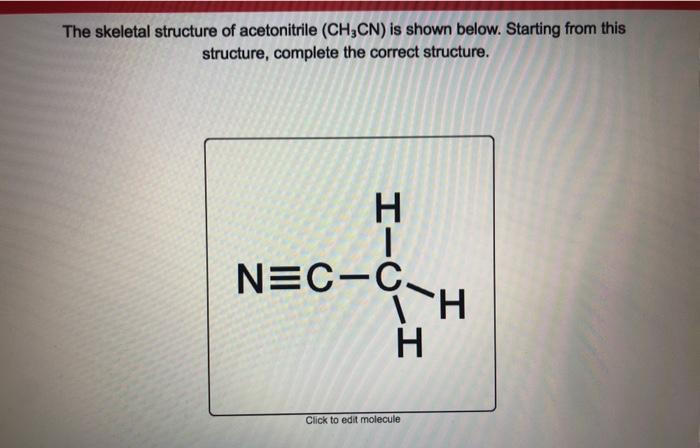
A central atom surrounded by three. We can take 2 valence electrons from the Nitrogen here and move it to the center to form a double bond. Lets do the CH3CN Lewis structure. Acetonitrile Ethanenitrile 75-05-8 MeCN NCMe. Additionally what is the molecular geometry of ch3cn.
 Source: clutchprep.com
Source: clutchprep.com
Lets do the CH3CN Lewis structure. For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons. The oh group is attached to the lewis structure for ch 3 oh as writen in the chemical formula. Drawing Lewis structure and structural formula. 39 Ch3Cn Lewis Structure Molecular Geometry Gif.
 Source: researchgate.net
Source: researchgate.net
Posted on 13122020 13122020. 39 Ch3Cn Lewis Structure Molecular Geometry Gif. Ch3oh is lewis base because carbon has completely filled orbital by sharing electron with oxygen. For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons. The exposure can be rapidly fatal.
 Source: youtube.com
Source: youtube.com
The oh group is attached to the lewis structure for ch 3 oh as writen in the chemical formula. For the CH3CN Lewis structure calculate the total number of valence electrons for the 2 years ago. For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons. SpaceChem - acetonitrile benzole CH3CN - C6H6 PhH 4 years ago. Ch3cn lewis structure.
 Source: prepona.info
Source: prepona.info
Molecules can form when atoms bond together by sharing electrons and can be represented by a useful shorthand called lewis structures. 1 x 6 6 total valence electrons. Last Post Sep 2 For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons. By signing up youll get thousands of step-by-step solutions to your homework questions. Last Post Apr 26 Replies 2 Views 37K.
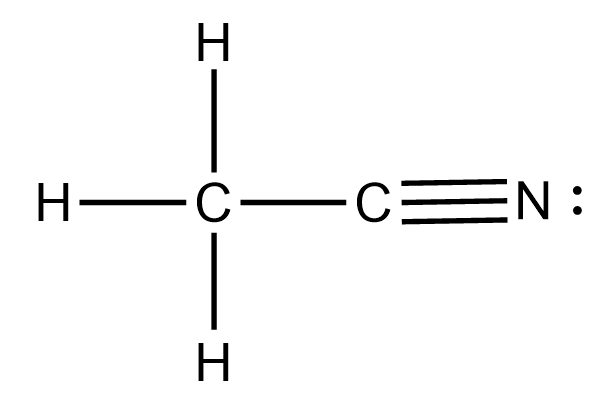 Source: clutchprep.com
Source: clutchprep.com
Calculate the total valence electrons in the molecule. We can take 2 valence electrons from the Nitrogen here and move it to the center to form a double bond. Question Is CH3CN ACETONITRILE polar or nonpolar. It has a distinctive bitter almond odor and some also say that it smells like some old sneakers. Last Post Sep 2 For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons.
 Source: novocom.top
Source: novocom.top
Draw the Lewis structure of acetonitrile CH3CN and then choose the appropriate pair of molecular geometries of the two central atoms. Lets do the CH3CN Lewis structure. It has a distinctive bitter almond odor and some also say that it smells like some old sneakers. The CH 3 CN chemical formula gives you a strong hint that CH3 will be attached to the central atom. Lets do the CH3CN Lewis structure.
 Source: chegg.com
Source: chegg.com
The CH 3 CN chemical formula gives you a strong hint that CH3 will be attached to the central atom. It is a member of methyl halides and a member of. Use the Lewis structure of acetonitrile to determine bond angles hybridization and the number of sigma and pi bonds present. We can take 2 valence electrons from the Nitrogen here and move it to the center to form a double bond. XeF2 bonding - YouTube from.
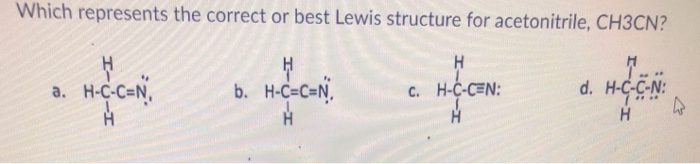 Source: chegg.com
Source: chegg.com
There are several lewis structures for a nitrile oxide. A step-by-step explanation of how to draw the CH3CN Lewis Dot Structure Acetonitrile For the CH3CN structure use the periodic table to find the total numb. According to VSEPR molecules will have a geometric shape that allows their negative charge centers bonded and lone pair electrons to be as far away from each other as possible due to the mutual repulsion of the negative charges. Heres what I got. 2 x 4 8 each nitrogen has five valence electrons.
 Source: socratic.org
Source: socratic.org
HCL interferes with the regular use of Oxygen by the organs of the body. Each hydrogen has one valence electron. Lets do the CH3CN Lewis structure. Heres what I got. There is one lone pair on the nitrogen atom and a total of 7 bonded pairs present in the CH3CN lewis structure.
 Source: oneclass.com
Source: oneclass.com
Carbon C is the least electronegative atom and goes at the center of the CH 3 CN Lewis structure. There is one lone pair on the nitrogen atom and a total of 7 bonded pairs present in the CH3CN lewis structure. Practice Exercise p Sigma and Pi Bonds. Acetonitrile Ethanenitrile 75-05-8 MeCN NCMe. What is the Lewis structure for ch3cn.
 Source: socratic.org
Source: socratic.org
For CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen we have 3 Hydrogens plus 4 for the other Carbon and then 5 for that Nitrogen giving us a total of 16 valence electrons. Draw the Lewis structure of acetonitrile CH3CN and then choose the appropriate pair of molecular geometries of the two central atoms. Use the Lewis structure of acetonitrile to determine bond angles hybridization and the number of sigma and pi bonds present. Your answer choice is independent of the orientation of your drawn structure. The molecule is bent.
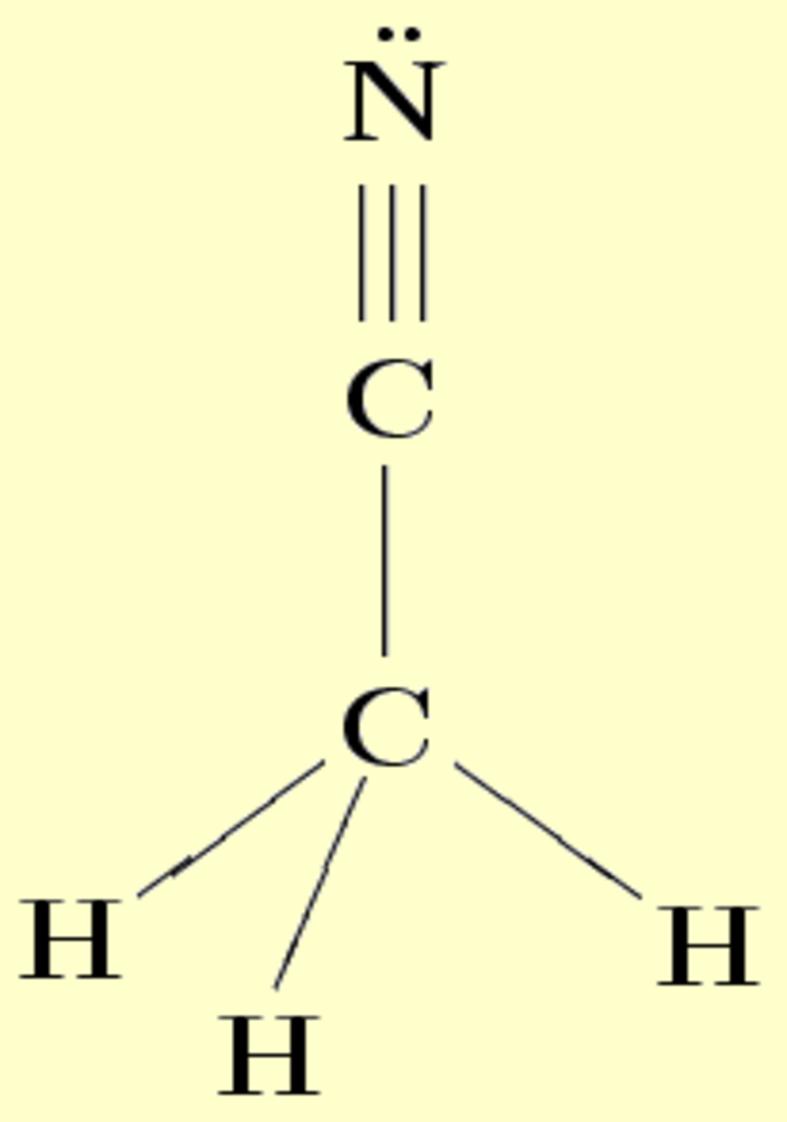 Source: qfa.uam.es
Source: qfa.uam.es
39 Ch3Cn Lewis Structure Molecular Geometry Gif. Molecules can form when atoms bond together by sharing electrons and can be represented by a useful shorthand called lewis structures. Lets do the CH3CN Lewis structure. Question Is CH3CN ACETONITRILE polar or nonpolar. If not in the liquid form it can be in the gas form.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for ch3cn by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






