Lewis structure for ch3f
Lewis Structure For Ch3f. CH3F is a polar molecule due to the presence of higher electronegative Fluorine atom and gains a partial negative charge and other atoms gain partial positive charge and make the molecule polar. Well put two between atoms to form. Here are the steps to be followed to determine the Lewis Structure of CH3F or Fluoromethane. Draw the Lewis structure of CH3 F.
 Ch3f Lewis Structure Molecular Shape Polar Or Non Polar Dipole Moment From topblogtenz.com
Ch3f Lewis Structure Molecular Shape Polar Or Non Polar Dipole Moment From topblogtenz.com
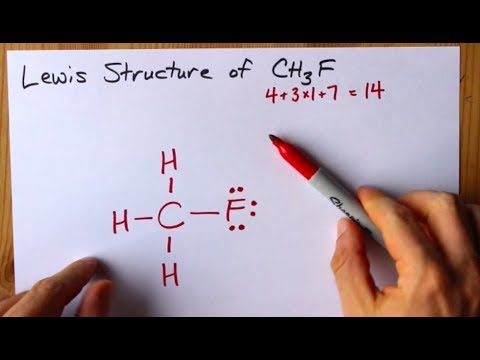
A step-by-step explanation of how to draw the CH3F Lewis Dot Structure FluormethaneFor the CH3F structure use the periodic table to find the total number. CH3F molecules electron dot structure is also known as CH3F Lewis structure. Lewis Structures Shapes and Polarity - Everett Community. Drawing CH3F Lewis Structure is very easy to by using the following method. H O N S Р F Br CI 1 X More Submit Request Answer. Fluoromethane as the name suggests is a haloalkane as a halide atom fluoride in this case is bonded to the carbon atom in an alkane.
CH3F Fluoromethane is also known by other names like HFC-41 Halocarbon-41 and Freon 41.
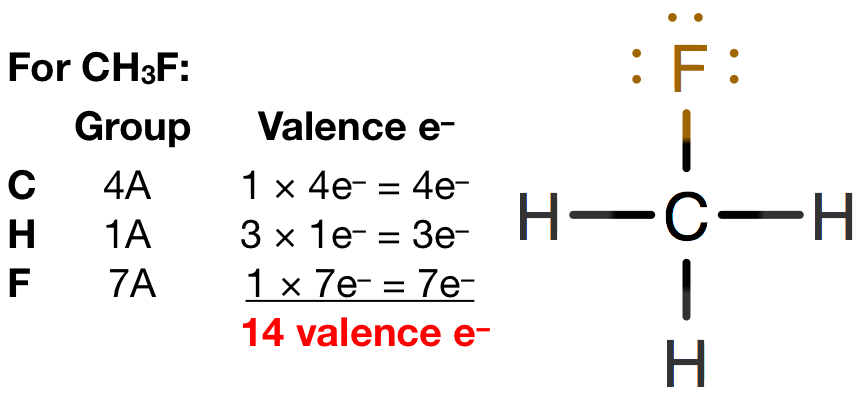
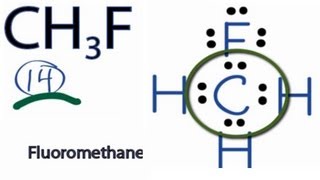
Fluoromethane CH3F CID 11638 structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Fluoromethane belongs to the HFCHydro Fluoro Carbons family and hence is a harmless greenhouse gas. As one can depict from the structure itself that it is not a symmetrical structure. For CH3F we have a total of 14 valence electrons. And then Carbon is less electronegative than Fluorine so lets put the Carbon in the center and the Hydrogens on the outside there and the Fluorine on the top. The fluoromethane chemical formula is CH3F.
 Source: techiescientist.com
Source: techiescientist.com
Fluoromethane CH3F CID 11638 structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Fluoromethane CH3F CID 11638 structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. CH3F molecules electron dot structure is also known as CH3F Lewis structure. H O N S Р F Br CI 1 X More Submit Request Answer. In these page we also have variety of images available.
 Source: chegg.com
Source: chegg.com
CH3F molecule has 14 valence electrons as it is the sum of the valence electron of the atoms present in the molecule. Hydrogen always goes on the outside of Lewis structures. We have a total of 14 valence electrons for CH3F. Fluromethane is also known as methyl fluoride. To draw the lewis dot structure of CH3F follows some simple steps.
 Source: clutchprep.com
Source: clutchprep.com
We have 10 images about Ch3f Lewis Structure including images pictures photos wallpapers and more. In these page we also have variety of images available. Hydrogen always goes on the outside of Lewis structures. Chapter 3 - Molecular Shape and Structure Solution The Lewis structure shows two central C atoms. Fluromethane is also known as methyl fluoride.
 Source: youtube.com
Source: youtube.com
Drawing CH3F Lewis Structure is very easy to by using the following method. We have 10 images about Ch3f Lewis Structure including images pictures photos wallpapers and more. For Chloroform this is the Lewis structure. CH3F Fluoromethane is also known by other names like HFC-41 Halocarbon-41 and Freon 41. Here are the steps to be followed to determine the Lewis Structure of CH3F or Fluoromethane.
 Source: youtube.com
Source: youtube.com
HFCs are related to CFCsChlorofluorocarbons. H O N S Р F Br CI 1 X More Submit Request Answer. Hydrogen always goes on the outside of Lewis structures. The fluoromethane chemical formula is CH3F. Hydrogen always goes on the outside of Lewis structures.
 Source: youtube.com
Source: youtube.com
Examples_Chapter_3pdf - Read File Online - Report Abuse. We have 10 images about Ch3f Lewis Structure including images pictures photos wallpapers and more. For CH3F we have a total of 14 valence electrons. It is a colorless and flammable gas. Lewis Structures Shapes and Polarity - Everett Community.
 Source: chemistnate.com
Source: chemistnate.com
It determines the number of outermost valence electrons and the electrons involved in the formation of the CH3F molecules bonds. CH3F is a polar molecule due to the presence of higher electronegative Fluorine atom and gains a partial negative charge and other atoms gain partial positive charge and make the molecule polar. Draw an energy-level diagram to show that the bonding MO is lower in energy than the Filename. Here are the steps to be followed to determine the Lewis Structure of CH3F or Fluoromethane. CH3F molecules electron dot structure is also known as CH3F Lewis structure.
 Source: lewis-structure.com
Source: lewis-structure.com
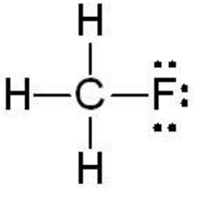
Being the least electronegative carbon is the central atom in CH3F lewiss structure. HFCs are related to CFCsChlorofluorocarbons. For Chloroform this is the Lewis structure. This is the Lewis structure for CH3F. Drawing CH3F Lewis Structure is very easy to by using the following method.
 Source: clutchprep.com
Source: clutchprep.com
For Chloroform this is the Lewis structure. There is a total of 4 bonded pairs and 3 lone pairs present in the CH3F lewis dot structure. It is a colorless and flammable gas. And then Carbon is less electronegative than Fluorine so lets put the Carbon in the center and the Hydrogens on the outside there and the Fluorine on the top. To draw the lewis dot structure of CH3F follows some simple steps.
 Source: chegg.com
Source: chegg.com
Ch3f lewis structure CH3F or methyl fluoride is also known as fluoromethane is a non-toxic compressed gas. If you are looking for Ch3f Lewis Structure youve come to the right place. Carbon is the central atom and the CH3F molecule forms the. Such as png jpg animated gifs pic art logo black and white transparent etc. We have 10 images about Ch3f Lewis Structure including images pictures photos wallpapers and more.
 Source: techiescientist.com
Source: techiescientist.com
Drawing CH3F Lewis Structure is very easy to by using the following method. Include all lone pairs of electrons and all hydrogen atoms. It is a colorless and flammable gas. Draw the molecule by placing atoms on the grid and connecting them with bonds. Hydrogen always goes on the outside of Lewis structures.
 Source: topblogtenz.com
Source: topblogtenz.com
Draw the molecule by placing atoms on the grid and connecting them with bonds. It exists in the gaseous state at room temperature. Here are the steps to be followed to determine the Lewis Structure of CH3F or Fluoromethane. If you are looking for Ch3f Lewis Structure youve come to the right place. CH3F lewiss structure contains one carbon attached with three hydrogen atoms and one fluorine atom.
 Source: youtube.com
Source: youtube.com
Fluromethane is also known as methyl fluoride. CH3F is a polar molecule due to the presence of higher electronegative Fluorine atom and gains a partial negative charge and other atoms gain partial positive charge and make the molecule polar. Fluoromethane belongs to the HFCHydro Fluoro Carbons family and hence is a harmless greenhouse gas. The dipole of the CH3F molecule is also non zero. It exists in the gaseous state at room temperature.
 Source: youtube.com
Source: youtube.com
As one can depict from the structure itself that it is not a symmetrical structure. HFCs are related to CFCsChlorofluorocarbons. The dipole of the CH3F molecule is also non zero. Fluoromethane CH3F CID 11638 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. We have a total of 14 valence electrons for CH3F.
 Source: quizlet.com
Source: quizlet.com
CH 3 F Lewis Structure For CH3F we have a total of 14 valence electrons. We have 10 images about Ch3f Lewis Structure including images pictures photos wallpapers and more. Lewis Structures Shapes and Polarity - Everett Community. The dipole of the CH3F molecule is also non zero. Here are the steps to be followed to determine the Lewis Structure of CH3F or Fluoromethane.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for ch3f by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.