Lewis structure for ch3no2
Lewis Structure For Ch3no2. C and N the central atoms. Nitromethane CH3NO2 CID 6375 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Lewis Structure of Methylamine CH3NH2 The Lewis structure is a diagrammatic representation of how the movement of valence electrons is occurring to ensure bond formation. H 1A 3 1 e 3 e.
 Part A Draw The Best Lewis Structure Of Ch3no2 A Chegg Com From chegg.com
Part A Draw The Best Lewis Structure Of Ch3no2 A Chegg Com From chegg.com
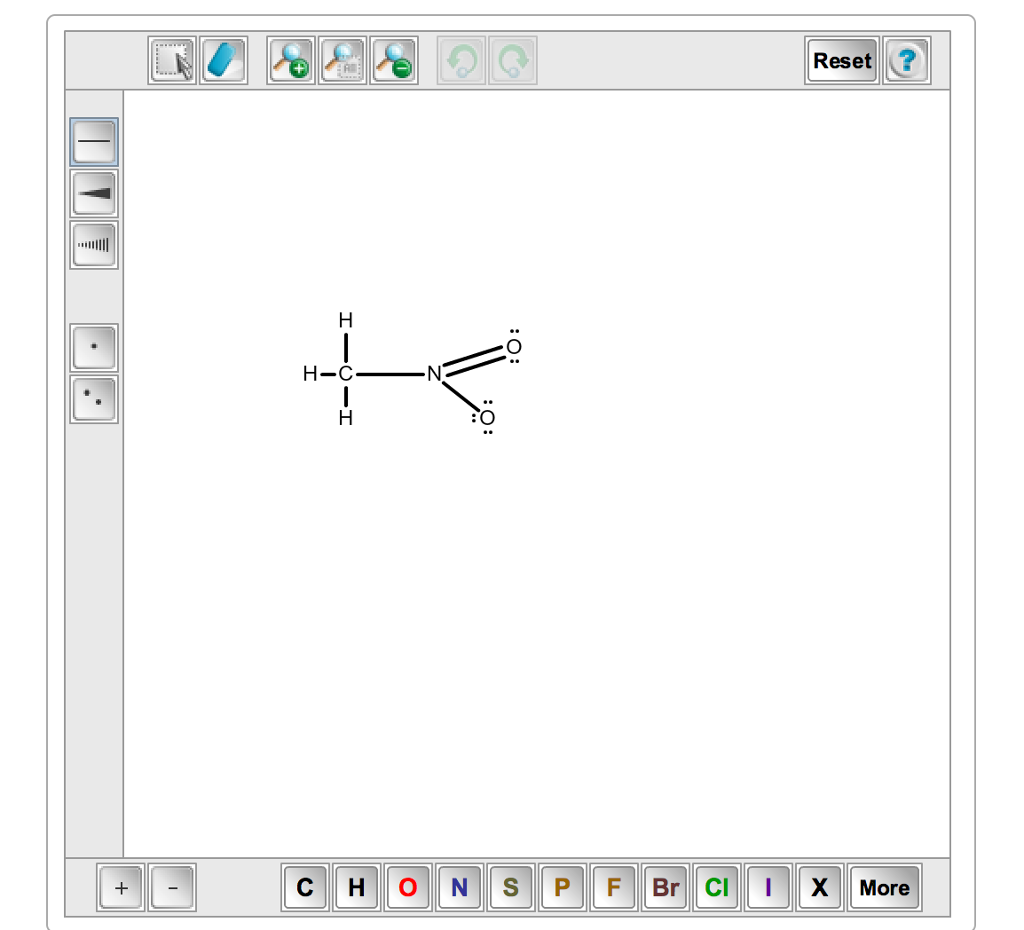
For the CH3NO2 Lewis structure we have a total of 24 valence electrons. Draw the best Lewis structure of CH3NO2 a neutral compound. Calculate the formal charge for each atom. Asked by wiki 18062021 in Chemistry viewed by 0 persons. Draw The Lewis Structure For CH3NO2 Include Re. ClCH2COONa NaNO2 H2O CH3NO2 NaCl NaHCO3 The Lewis Structure of Nitromethane CH3NO2 In order to start with the Lewis structure of nitromethane it is crucial to study the individual participating atoms first.
Well form bonds between atoms and each one of these bonds represents two electrons.
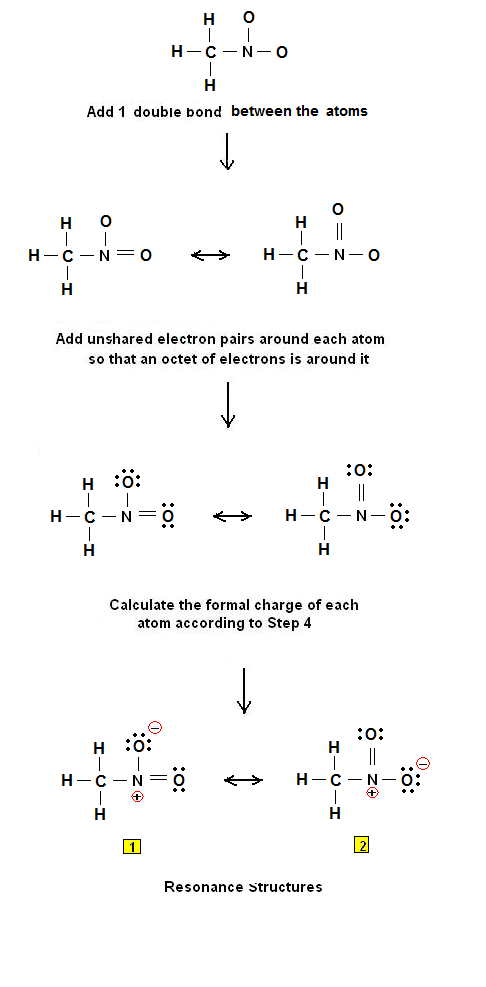
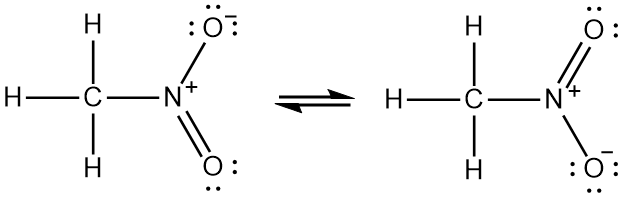
Draw the Lewis structure of nitromethane CH3NO2 clearly indicating resonance contributors as well as non-bonding pairs of electrons and formal charges as relevant. Were being asked to draw the Lewis structure for CH3NO2. Asked by wiki 18062021 in Chemistry viewed by 0 persons. These resonance structures. Draw the Lewis structure by placing atoms on the grid and connecting them with bonds. A Nitromethane Is An Organic Compound With The M.
 Source: chegg.com
Source: chegg.com
Draw the Lewis structure of nitromethane CH3NO2 clearly indicating resonance contributors as well as non-bonding pairs of electrons and formal charges as relevant. ClCH2COONa NaNO2 H2O CH3NO2 NaCl NaHCO3 The Lewis Structure of Nitromethane CH3NO2 In order to start with the Lewis structure of nitromethane it is crucial to study the individual participating atoms first. For the CH3NO2 Lewis structure we have a total of 24 valence electrons. For this we need to do the following steps. Were being asked to draw the Lewis structure for CH3NO2.
 Source: techiescientist.com
Source: techiescientist.com
The structure in Step 1 has one double bond. Lewis Structure of Methylamine CH3NH2 The Lewis structure is a diagrammatic representation of how the movement of valence electrons is occurring to ensure bond formation. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions as a reaction medium and as a cleaning solvent. It is the simplest organic nitro compound. A step-by-step explanation of how to draw the CH3NO2 Lewis Dot StructureThere are several ways to draw the CH3NO2 Lewis structure.
 Source: techiescientist.com
Source: techiescientist.com
Draw the curved arrows that shows conversion from one resonance structure to another. Lewis Structure of Methylamine CH3NH2 The Lewis structure is a diagrammatic representation of how the movement of valence electrons is occurring to ensure bond formation. Draw the curved arrows that shows conversion from one resonance structure to another. Draw the Lewis structure of nitromethane CH3NO2 clearly indicating resonance contributors as well as non-bonding pairs of electrons and formal charges as relevant. It is the simplest organic nitro compound.
 Source: brainly.com
Source: brainly.com
Latest questions in Chemistry. Were being asked to draw the Lewis structure for CH3NO2. Include all lone pairs of electrons and nonzero formal charges. Finally draw a resonance hybrid for nitromethane CH3NO2. NH2- Lewis Structure Molecular Geometry Hybridization Polarity.
 Source: youtube.com
Source: youtube.com
Nitromethane CH3NO2 CID 6375 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. There are two possible Lewis structures for CH 3 NO 2. These resonance structures. The structure in Step 1 has one double bond. For the CH3NO2 Lewis structure we have a total of 24 valence electrons.
 Source: chem-net.blogspot.com
Source: chem-net.blogspot.com
Finally draw a resonance hybrid for nitromethane CH3NO2. The atomic number of Carbon is 6 which makes it electronic configuration 1s2 2s2 2p2. These resonance structures. For this we need to do the following steps. Nitromethane sometimes shortened to simply nitro is an organic compound with the chemical formula CH 3NO 2.
 Source: youtube.com
Source: youtube.com
Well form bonds between atoms and each one of these bonds represents two electrons. Include all lone pairs of electrons and nonzero formal charges. Nitromethane sometimes shortened to simply nitro is an organic compound with the chemical formula CH 3NO 2. Do you know the better answer. Asked by wiki 18062021 in Chemistry viewed by 0 persons.
 Source: clutchprep.com
Source: clutchprep.com
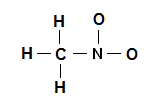
Each of these isomers. C and N the central atoms. Draw The Lewis Structure For CH3NO2 Include Re. To study a chemical compound the Lewis structure is the first step to begin. Where V 1 1 1 4 5 6 6 24 Therefore P 6n 2 V 6 4 2 24 2 Therefore there is one double bond.
 Source: chem-net.blogspot.com
Source: chem-net.blogspot.com
N 1A 1. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions as a reaction medium and as a cleaning solvent. There are two possible Lewis structures for CH 3 NO 2. The atomic number of Carbon is 6 which makes it electronic configuration 1s2 2s2 2p2. C 4A 1 4 e 4 e.
 Source: clutchprep.com
Source: clutchprep.com
By signing up youll get. To study a chemical compound the Lewis structure is the first step to begin. A Lewis base is a donor molecule that easily donates a pair of non-bonding electrons to achieve a stable electronic configuration. C 4A 1 4 e 4 e. Lewis Structure of Methylamine CH3NH2 The Lewis structure is a diagrammatic representation of how the movement of valence electrons is occurring to ensure bond formation.
 Source: clutchprep.com
Source: clutchprep.com
N 1A 1. A Lewis base is a donor molecule that easily donates a pair of non-bonding electrons to achieve a stable electronic configuration. Where n in this case is 4 since CH3NO2 consists of seven atoms but three of them are H atoms. And then well go around the outside of the Oxygens to fill their octets. Calculate the total number of valence electrons present.
 Source: clutchprep.com
Source: clutchprep.com
A Lewis base is a donor molecule that easily donates a pair of non-bonding electrons to achieve a stable electronic configuration. NH2- Lewis Structure Molecular Geometry Hybridization Polarity. It is the simplest organic nitro compound. Nitromethane CH3NO2 CID 6375 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. C and N the central atoms.
 Source: study.com
Source: study.com
Draw the Lewis structure for the molecule. These resonance structures. So we have 12 14 and 24. Do you know the better answer. Calculate the formal charge for each atom.
 Source: techiescientist.com
Source: techiescientist.com
Were being asked to draw the Lewis structure for CH 3 NO 2. Draw the Lewis dot structure for CH3NO2. Latest questions in Chemistry. It is the simplest organic nitro compound. Where n in this case is 4 since CH3NO2 consists of seven atoms but three of them are H atoms.
 Source: youtube.com
Source: youtube.com
Where V 1 1 1 4 5 6 6 24 Therefore P 6n 2 V 6 4 2 24 2 Therefore there is one double bond. C 4A 1 4 e 4 e. Determine the central atom in this molecule. These resonance structures. Lewis Structure of Methylamine CH3NH2 The Lewis structure is a diagrammatic representation of how the movement of valence electrons is occurring to ensure bond formation.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for ch3no2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.