Lewis structure for h2so3
Lewis Structure For H2so3. H2so3 Lewis Structure Fobiaspoleczna Info Steric Number A Focus Into The Key To The Vsepr Theory Lewis Structure H2so4 Untitled Bond Angles Chemistry Video Clutch Prep Videos Matching H2so4 Lewis Structure How To Draw The Lewis These Species Do Not Obey The Octet Rule Draw A Lewis Exercise Vsepr Theory And The Shapes Of Molecules 10 1 Lewis Structures And The. When drawing Lewis dot structure count the valence electrons of each element in the structure and the total valence electrons of the compound. Write Lewis structure for H2SO3 H is bonded to O. Sulfurous acid-Lewis Structure H 2 SO 3 is also called sulfur dioxide solution or Dihydrogen tri-oxosulfate or Tri-oxosulfuric acid.
 Lewis Dot Structure Of H2so3 Novocom Top From novocom.top
Lewis Dot Structure Of H2so3 Novocom Top From novocom.top
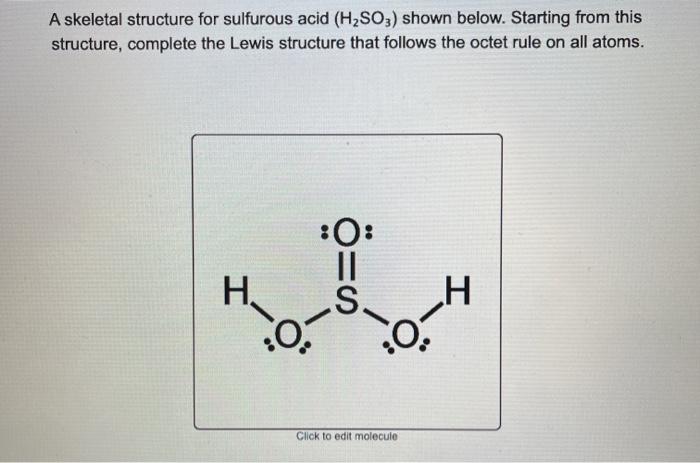
If playback doesnt begin shortly try. Answered Lewis dot structure of H2SO3 1 See answer gd4122014 is waiting for your help. Complete octets on outside atoms. Sulfurous acid-Lewis Structure H 2 SO 3 is also called sulfur dioxide solution or Dihydrogen tri-oxosulfate or Tri-oxosulfuric acid. For the Lewis structure for H 2 SO 3 you should take formal charges into account to find the best Lewis structure for the molecule. Therefore the Lewis structure of H2SeO3.
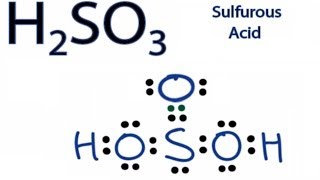
Lewis Dot Structure of H2SO3 How to Draw Lewis Structures Class 11 Chemistry Chemical Bonding - YouTube.
So the total number of valence electrons is calculated as. Include all lone pairs of electrons. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. H always goes outside. A step-by-step explanation of how to draw the H2SO3 Lewis Structure Sulfurous acid. Sulfurous acid-Lewis Structure H 2 SO 3 is also called sulfur dioxide solution or Dihydrogen tri-oxosulfate or Tri-oxosulfuric acid.
 Source: brainly.in
Source: brainly.in
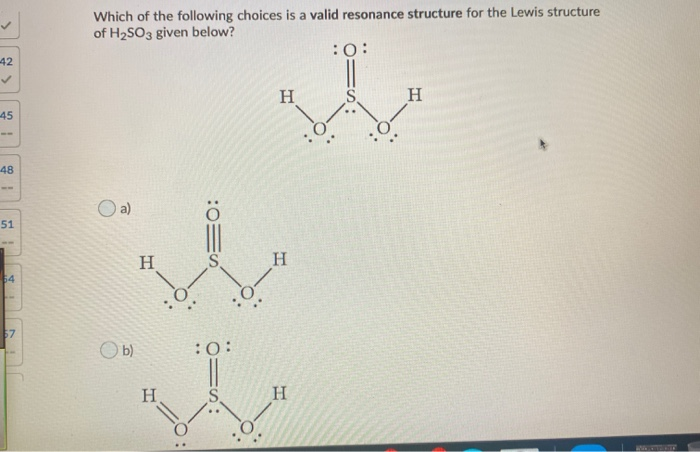
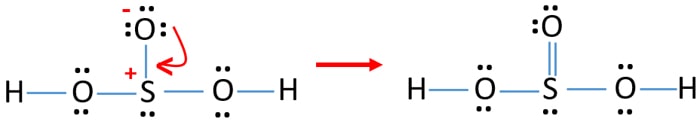
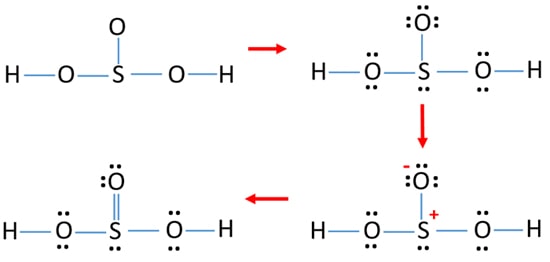
Definition Lewis Structure. In the Lewis structure for H 2 SO 3 there are a total of 26 valence electrons. When we have an H or H2 in front of a polyatomic molecule like CO3. Complete octets on outside atoms. For H 2 SO 4 molecule Total pairs of electrons are 16.
 Source: study.com
Source: study.com
Include all lone pairs of electrons. Find the total valence electrons for the molecule. As per rule the Sulfur atom with. Lewis Dot Structure of H2SO3 How to Draw Lewis Structures Class 11 Chemistry Chemical Bonding - YouTube. This lesson will help you learn about.
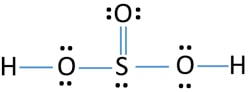 Source: chemistryscl.com
Source: chemistryscl.com
Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. A step-by-step explanation of how to draw the H2SO3 Lewis Structure Sulfurous acid. Write Lewis structure for H2SO3 H is bonded to O. When drawing Lewis dot structure count the valence electrons of each element in the structure and the total valence electrons of the compound. Put two electrons between atoms to form a chemical bond.
 Source: chegg.com
Source: chegg.com
Include all lone pairs of electrons. Therefore the Lewis structure of H2SeO3. Total electron pairs are determined by dividing the number total valence electrons by two. Gd4122014 gd4122014 09102018 Chemistry Secondary School 6 pts. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells.
 Source: chemistryscl.com
Source: chemistryscl.com
When we have an H or H2 in front of a polyatomic molecule like CO. Write Lewis structure for H2SO3 H is bonded to O. Complete octets on outside atoms. In H2SeO3 H 2 S e O 3 there are three oxygen atoms one selenium atom and two hydrogen atoms. If playback doesnt begin shortly try.
 Source: youtube.com
Source: youtube.com
Definition Lewis Structure. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. In H2SeO3 H 2 S e O 3 there are three oxygen atoms one selenium atom and two hydrogen atoms. This is the H2SO3 Lewis structure. Sulfurous acid-Lewis Structure H 2 SO 3 is also called sulfur dioxide solution or Dihydrogen tri-oxosulfate or Tri-oxosulfuric acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Include all hydrogen atoms. When we have an H or H2 in front of a polyatomic molecule like CO3. Further instruction about this subject is available to you in the lesson titled H2SO3. Definition Lewis Structure. If playback doesnt begin shortly try.
 Source: chemistryscl.com
Source: chemistryscl.com
This lesson will help you learn about. See the Big List of Lewis Structures. Include all lone pairs of electrons. A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure Carbonic AcidFor the H2CO3 structure use the periodic table to find the total numb. Put two electrons between atoms to form a chemical bond.
 Source: novocom.top
Source: novocom.top
In H2SeO3 H 2 S e O 3 there are three oxygen atoms one selenium atom and two hydrogen atoms. Complete octets on outside atoms. Find an answer to your question Lewis dot structure of H2SO3 1. For H 2 SO 4 molecule Total pairs of electrons are 16. Find the total valence electrons for the molecule.
 Source: whatsinsight.org
Source: whatsinsight.org
In the Lewis structure for H 2 SO 3 there are a total of 26 valence electrons. If playback doesnt begin shortly try. H has 1 valence electron S has 6 valence electrons and O has 6 valence. A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure Carbonic AcidFor the H2CO3 structure use the periodic table to find the total numb. For H 2 SO 4 molecule Total pairs of electrons are 16.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Sulfurous Acid H 2 SO 3 Lewis Structure. If playback doesnt begin shortly try. Put two electrons between atoms to form a chemical bond. Definition Lewis Structure. H always goes outside.
 Source: study.com
Source: study.com
Write Lewis structure for H2SO3 H is bonded to O. A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure Carbonic AcidFor the H2CO3 structure use the periodic table to find the total numb. Write Lewis structure for H2SO3 H is bonded to O. Steps for Writing Lewis Structures. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3.
This is the H2SO3 Lewis structure. Add your answer and earn points. Include all lone pairs of electrons. Sulfurous acid-Lewis Structure H 2 SO 3 is also called sulfur dioxide solution or Dihydrogen tri-oxosulfate or Tri-oxosulfuric acid. H has 1 valence electron S has 6 valence electrons and O has 6 valence.
 Source: clutchprep.com
Source: clutchprep.com
H2so3 Lewis Structure Fobiaspoleczna Info Steric Number A Focus Into The Key To The Vsepr Theory Lewis Structure H2so4 Untitled Bond Angles Chemistry Video Clutch Prep Videos Matching H2so4 Lewis Structure How To Draw The Lewis These Species Do Not Obey The Octet Rule Draw A Lewis Exercise Vsepr Theory And The Shapes Of Molecules 10 1 Lewis Structures And The. Find the total valence electrons for the molecule. Total electron pairs are determined by dividing the number total valence electrons by two. H always goes outside. This is the H2SO3 Lewis structure.
 Source: youtube.com
Source: youtube.com
A step-by-step explanation of how to draw the H2SO3 Lewis Structure Sulfurous acid. When we have an H or H2 in front of a polyatomic molecule like CO. Also there is one lone pairs on sulfur atom. In the Lewis structure for H 2 SO 3 there are a total of 26 valence electrons. Total electron pairs are determined by dividing the number total valence electrons by two.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for h2so3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.