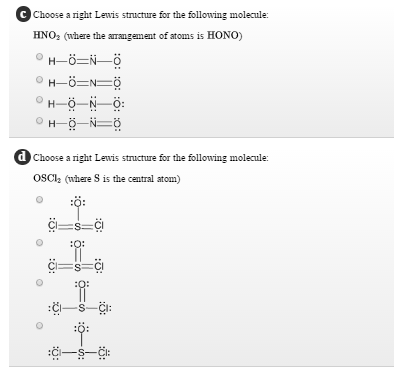
Lewis structure for hono
Lewis Structure For Hono. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. H Group 1A 1 ve. Please note none of the solutions are using the expanded octet rule or formal charges H 2. A variety of articles about lewis structure for hono have been classified well.
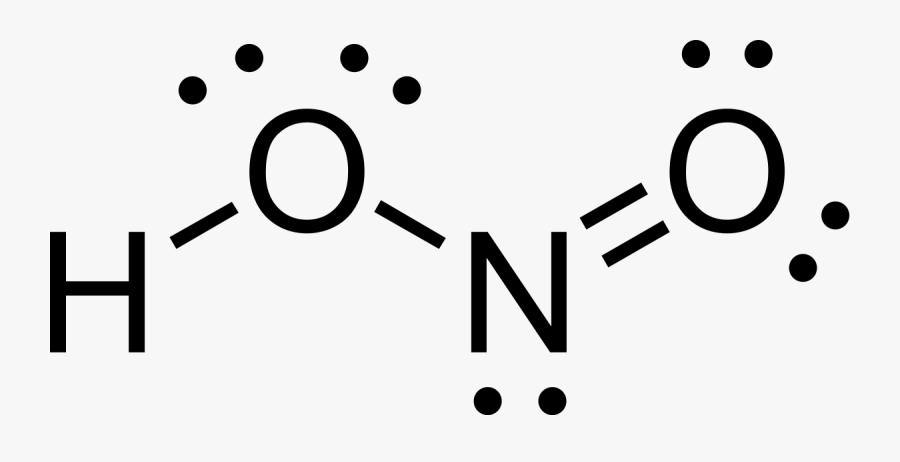 Clip Art Hono Lewis Structure Nitrous Acid Free Transparent Clipart Clipartkey From clipartkey.com
Clip Art Hono Lewis Structure Nitrous Acid Free Transparent Clipart Clipartkey From clipartkey.com
Predict the shape of the molecules of the compound. A Write a Lewis structure. H 2 CCH 2. Write Lewis structures for the following. A variety of articles about lewis structure for hono have been classified well. Get the detailed answer.
Write the Lewis structure for each molecule.
B What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO 2 molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. And then because it has a three minus charge there are an additional three valence electrons for a total of 32 Valence electrons. There are no charges on atoms and one double bond exists between nitrogen and one oxygen atom in the lewis structure of nitrous acid. N Group 5A 5 ve. H bonded to one of the O atoms d SO2.
 Source: clipartkey.com
Source: clipartkey.com
Consider nitrous acid mathrmHNO_2 HONO. Chemistry Chemistry by OpenStax 2015-05-04 Consider nitrous acid HNO 2 HONO. Predict the shape of the molecules of the compound. Made with Explain Everything. We are asked to write a Lewis structure for HONO.
 Source: youtube.com
Source: youtube.com
We are asked to write a Lewis structure for HONO. A Write a Lewis structure. HNO2Nitrous acid Lewis Structure HNO2Nitrous acidlewis stricture is drawn step by step by using total valence electrons of each element. Lewis Structure For Hono. NO N 2.
 Source: study.com
Source: study.com
Oxygen has six but there are four of them. First one is peel 43 minus phosphorus has five valence electrons. H 2 CCH 2. Steps of drawing lewis structure. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons.
 Source: clker.com
Source: clker.com
Get the detailed answer. Consider nitrous acid mathrmHNO_2 HONO. H 2 CCH 2. Write Lewis structures for the following. N Group 5A 5 ve.
 Source: youtube.com
Source: youtube.com
Lewis structure practice docx lewis structure practice a nf3 b clo3 c hobr d so32e cs2 f bf4 g which is the least polar bond a ocl b sf c pcl d cf e ncl 9 how many valence chem133 week 11 forum pdf 18 consider nitrous acid hno2honoa write a lewis structureb diagrama de roberts doc draw a diagram illustrating the theoretical picture of. B What are the electron pair and molecular geometries of the internal oxygen and. Chemistry Chemistry by OpenStax 2015-05-04 Consider nitrous acid HNO 2 HONO. N Group 5A 5 ve. Get the detailed answer.
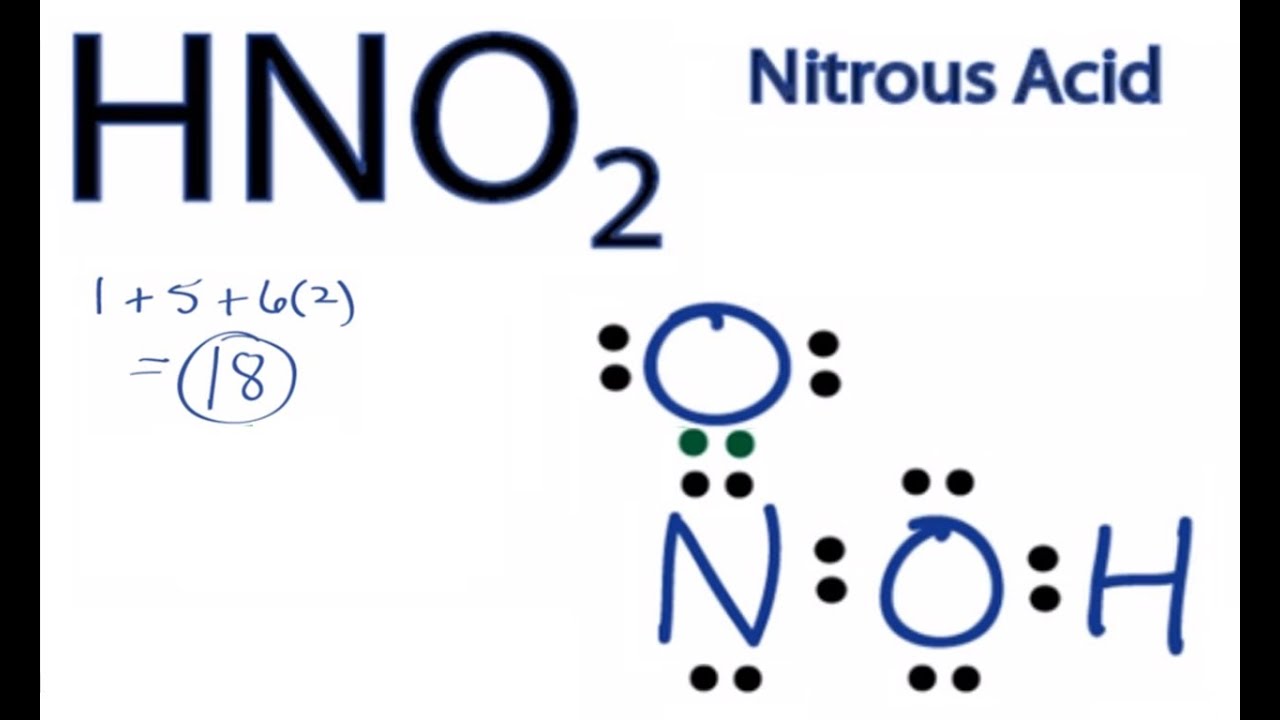 Source: youtube.com
Source: youtube.com
To draw the Lewis structures for these poly atomic ions we first need to sum up the total number of valence electrons for each Polly Atomic ion. What is the difficulty of this problem. We are asked to write a Lewis structure for HONO. HOFO Lewis Structure. Steps of drawing lewis structure of hno 3 there are.
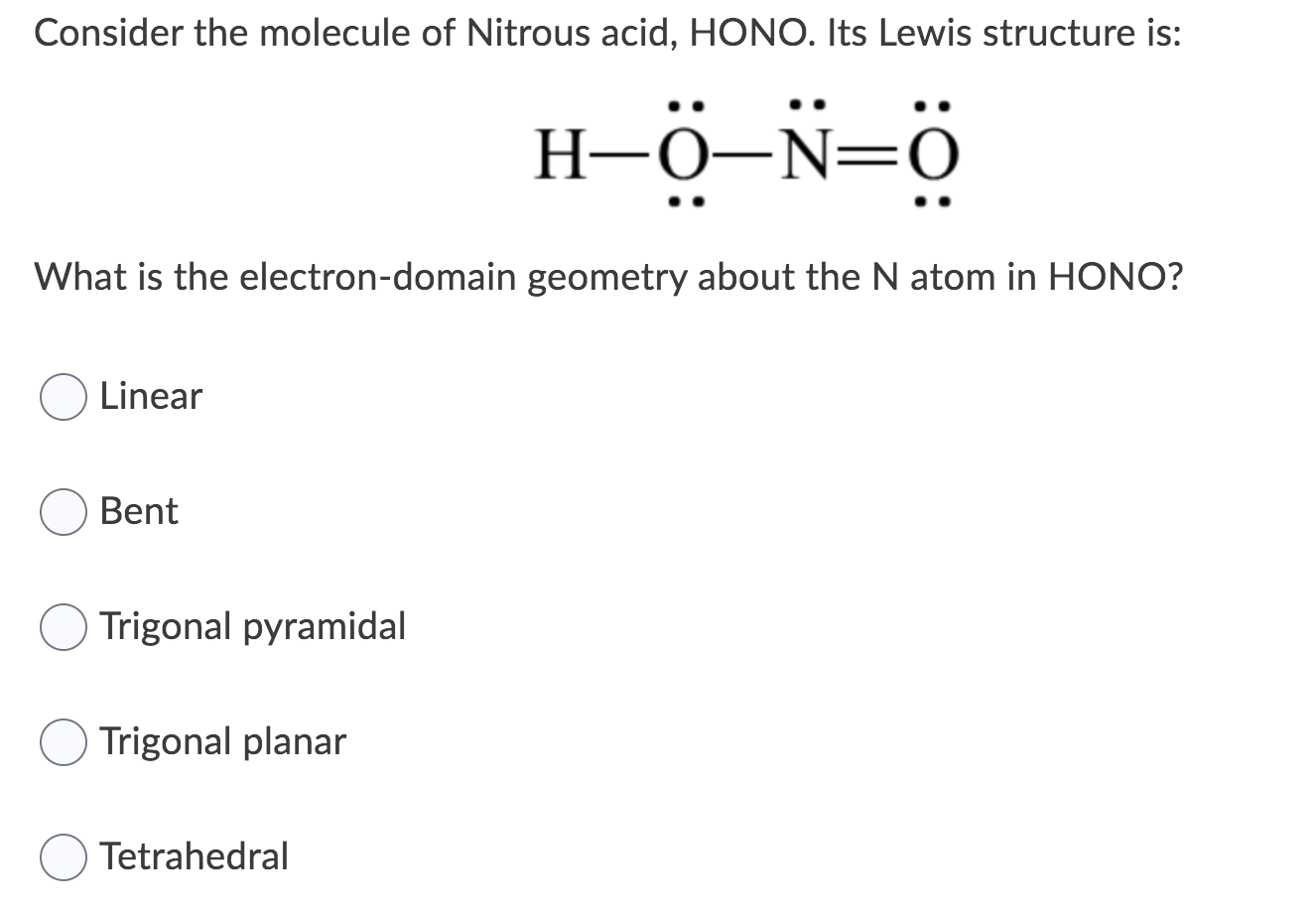
The Lewis structure of SO2 3 S O 3 2 is shown below. B What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO 2 molecule. To draw the Lewis structures for these poly atomic ions we first need to sum up the total number of valence electrons for each Polly Atomic ion. N Group 5A 5 ve. A Write a Lewis structure.
 Source: chegg.com
Source: chegg.com
Lewis structure practice docx lewis structure practice a nf3 b clo3 c hobr d so32e cs2 f bf4 g which is the least polar bond a ocl b sf c pcl d cf e ncl 9 how many valence chem133 week 11 forum pdf 18 consider nitrous acid hno2honoa write a lewis structureb diagrama de roberts doc draw a diagram illustrating the theoretical picture of. N Group 5A 5 ve. A O2 b CO c HONO N is central. Get the detailed answer. A Write a Lewis structure.
 Source: youtube.com
Source: youtube.com
A step-by-step explanation of how to draw the HONO Lewis Dot StructureFor the HONO Lewis structure calculate the total number of valence electrons for the. We are asked to write a Lewis structure for HONO. And then because it has a three minus charge there are an additional three valence electrons for a total of 32 Valence electrons. Predict the shape of the molecules of the compound. A variety of articles about lewis structure for hono have been classified well.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Steps of drawing lewis structure of hno 3 there are. The lewis structure of molecule is given as follows. The Lewis structure of SO2 3 S O 3 2 is shown below. CN Answer a. Write the lewis structure for a molecule of the compound.
 Source: youtube.com
Source: youtube.com
H bonded to one of the O atoms d SO2. H Group 1A 1 ve. And then because it has a three minus charge there are an additional three valence electrons for a total of 32 Valence electrons. Chemistry Chemistry by OpenStax 2015-05-04 Consider nitrous acid HNO 2 HONO. Get the detailed answer.
 Source: vedantu.com
Source: vedantu.com
Lewis Structure For Hono. What is the difficulty of this problem. And then because it has a three minus charge there are an additional three valence electrons for a total of 32 Valence electrons. C What is the hybridization on the internal oxygen and nitrogen atoms in HNO 2. Steps of drawing lewis structure of hno 3 there are.
 Source: redbubble.com
Source: redbubble.com
N Group 5A 5 ve. Echemi shares information about hono lewis structure. HNO2Nitrous acid Lewis Structure HNO2Nitrous acidlewis stricture is drawn step by step by using total valence electrons of each element. Hence it will share its electron with oxygen atom and form double bond with one oxygen atom and single bond with other 2 oxygen atoms. A step-by-step explanation of how to draw the HOCl Lewis Structure Hypochlorous AcidBecause HOCl is an acid well put the Hydrogen atom on the outside of.
 Source: novocom.top
Source: novocom.top
Total 19 ve. And then because it has a three minus charge there are an additional three valence electrons for a total of 32 Valence electrons. Made with Explain Everything. A variety of articles about lewis structure for hono have been classified well. First one is peel 43 minus phosphorus has five valence electrons.
 Source: en.wikipedia.org
Source: en.wikipedia.org
H bonded to one of the O atoms d SO2. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. Chemistry Chemistry by OpenStax 2015-05-04 Consider nitrous acid HNO 2 HONO. HOFO Lewis Structure. A step-by-step explanation of how to draw the HOFO Lewis Dot StructureFor the HOFO structure use the periodic table to.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for hono by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.