Lewis structure for n2h2
Lewis Structure For N2h2. N2H2 Lewis Structure Dinitrogen Dihydride Today in this video we help you determine the Lewis Structure of Dinitrogen Dihydride. Physics draw the lewis structure for n2h2 a neutral molecule 9752 results science draw the lewis structure for n2h2 a neutral molecule. Hydrogen is in group 1. This is the N2H2 Lewis structure.
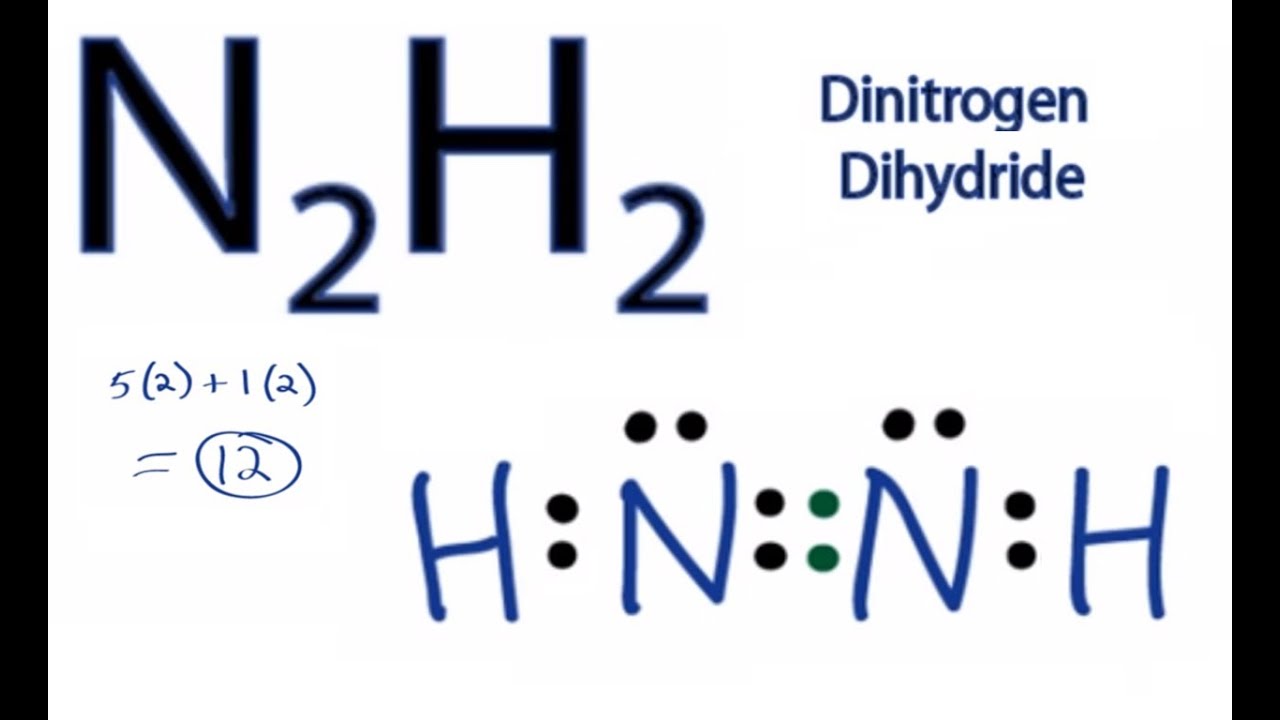 N2h2 Lewis Structure How To Draw The Lewis Structure For Dinitrogen Dihydride Youtube From youtube.com
N2h2 Lewis Structure How To Draw The Lewis Structure For Dinitrogen Dihydride Youtube From youtube.com
In the n 2 h 2 lewis structure the two nitrogen n atoms go in the center hydrogen always goes on the outside. N2H2 is straightforward with no double or triple bonds. This is the N2H2 Lewis structure. Hydrogen is in group 1. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The Lewis structure of Diimide N₂H₂ is shown below.
After that we can put an atom of Hydrogen each on.
This is the N2H2 Lewis structure. In part they were given and to H two So nitrogen will give a total of 10 valence electrons to from hydrogen for a total of 12 valence electrons for molecule. Lewis structure for n2h2. In the N2H2 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Option-C each nitrogen has one nonbinding electron pair is the correct answer. Each hydrogen has one nonbonding electron pair lone pair FREE Expert Solution Show answer.
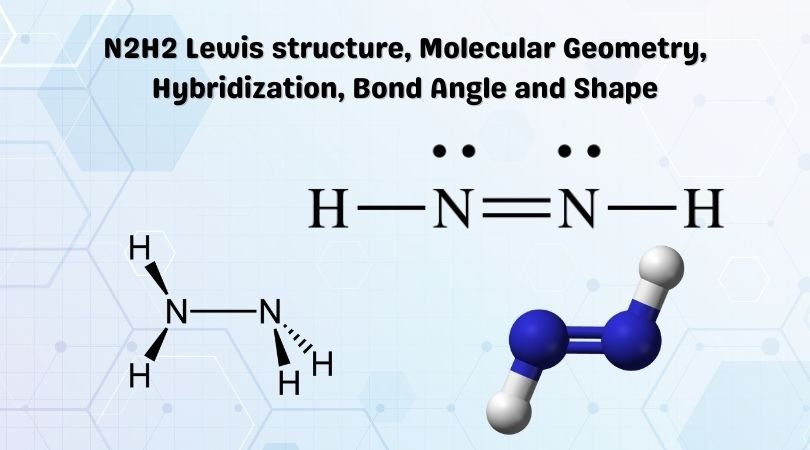 Source: geometryofmolecules.com
Source: geometryofmolecules.com
In the Lewis structure for N2H2 there are a. Hydrogen is in group 1. Each nitrogen has one nonbonding electron pair lone-paire. Each hydrogen has one nonbonding electron pair lone pair FREE Expert Solution Show answer. Lewis structure for n2h2.
 Source: novocom.top
Source: novocom.top
Lewis structure for n2h2. Each nitrogen has one nonbonding electron pair lone-paire. In the N 2 H 2 Lewis. N 2 H 2 is straightforward with no double or triple bonds. Lewis structure for n2h2.
 Source: quora.com
Source: quora.com
Hydrogen is in group 1. N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. Each hydrogen has one nonbonding electron pair lone pair FREE Expert Solution Show answer. It is the conjugate acid of a diazenideN2H2 Lewis structure Molecular Geometry Hybridization Bond Angle and Shape. In the Lewis.
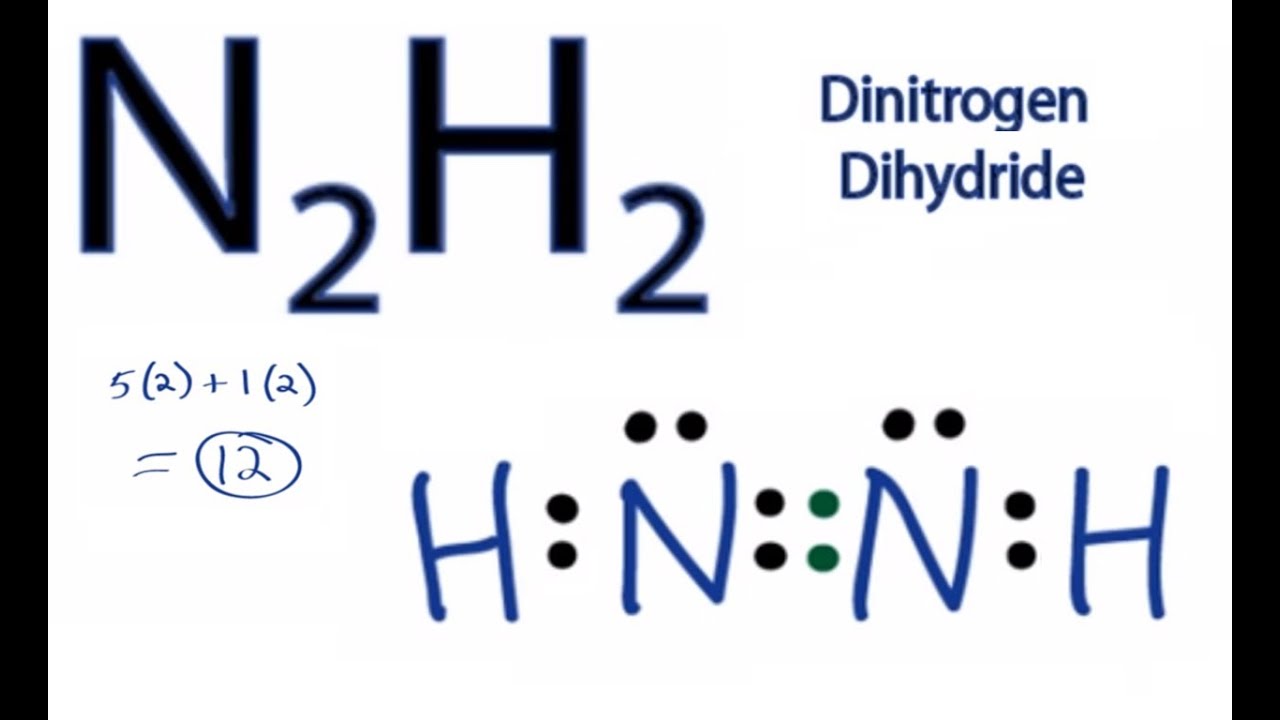 Source: youtube.com
Source: youtube.com
N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. Read Online How To Draw Lewis Dot Structure For N2h2 U Can. Check out a sample QA here. There is a nitrogen-nitrogen triple bondb. Diazene N2H2 or H2N2 CID 123195 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
 Source: clutchprep.com
Source: clutchprep.com
Q9 6 PtsYT He Lewis Structure For N2H2. 98 103 ratings Problem Details. Diazene N2H2 or H2N2 CID 123195 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Physics draw the lewis structure for n2h2 a neutral molecule 9752 results science draw the lewis structure for n2h2 a neutral molecule. Asked by amy on december 3 2012 chemistry draw the best lewis structure for ncch2coch2cho a neutral molecule.
 Source: techiescientist.com
Source: techiescientist.com
Check out a sample QA here. The Lewis structure of Diimide N₂H₂ is shown below. In part they were given and to H two So nitrogen will give a total of 10 valence electrons to from hydrogen for a total of 12 valence electrons for molecule. Each hydrogen has one nonbonding electron pair lone pair FREE Expert Solution Show answer. Each nitrogen has one nonbonding electron pair lone-paire.
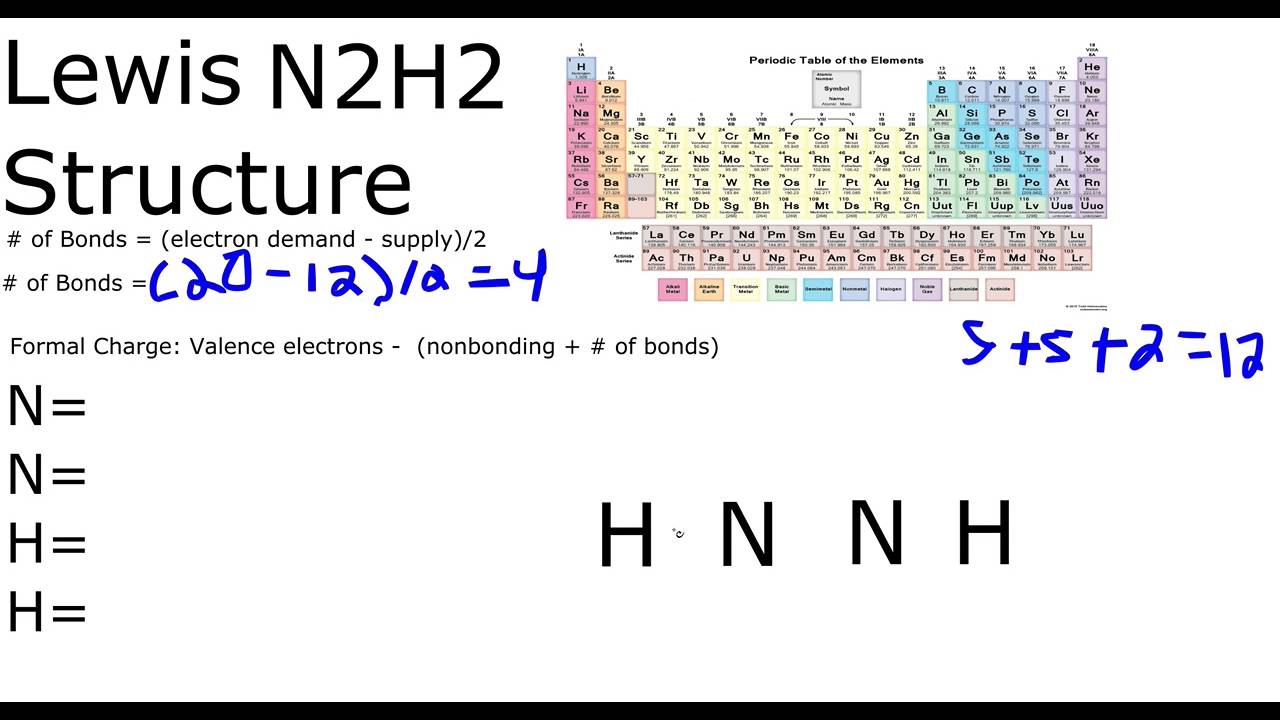 Source: youtube.com
Source: youtube.com
After that we can put an atom of Hydrogen each on. In the Lewis structure for N2H2 there are a. Diazene N2H2 or H2N2 CID 123195 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Lewis dot structure for n2h2 The Lewis structure of Diimide N₂H₂ is shown belowIn this molecule two Nitrogen atoms attached to each other through a double bond are further attached to one one Hydrogen atom. Hydrogen atoms always go on the outside so lets put the two Nitrogen atoms in the center.
 Source: techiescientist.com
Source: techiescientist.com
A Lewis structure is a pictorial representation of the arrangement of atoms in a molecule. Check out a sample QA here. Option-C each nitrogen has one nonbinding electron pair is the correct answer. A Lewis structure is a pictorial representation of the arrangement of atoms in a molecule. Nitrogen is in Group 5 or fifteen on the periodic table so it has five valence electrons but we have two of them.
 Source: study.com
Source: study.com
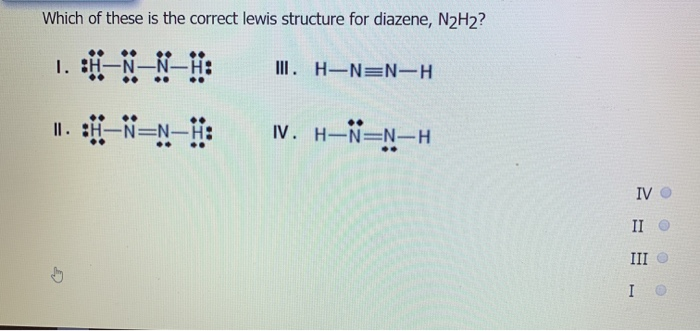
Lewis structure for n2h2. Lewis structure for n2h2. N2h2 lewis structure how to draw the for dinitrogen. Each nitrogen has one nonbonding electron pair lone-paire. A a nitrogen-nitrogen triple bond B a nitrogen-nitrogen single bond C each nitrogen has one nonbonding electron pair D each nitrogen has two nonbonding electron pairs E each hydrogen has one nonbonding electron pair.
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
In the Lewis. N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. N2h2 lewis structure molecular geometry hybridization and mo diagram dinitrogen dihydride has the chemical formula of n2h2. In the n 2 h 2 lewis structure the two nitrogen n atoms go in the center hydrogen always goes on the outside. Hydrogen is in group 1.
 Source: techiescientist.com
Source: techiescientist.com
Diazene N2H2 or H2N2 CID 123195 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Lewis Structures Practice Worksheet 30 PPs. Hydrogen is in group 1. A Lewis structure is a pictorial representation of the arrangement of atoms in a molecule. In the Lewis structures of N2H2a.
 Source: youtube.com
Source: youtube.com
Asked by amy on december 3 2012 chemistry draw the best lewis structure for ncch2coch2cho a neutral molecule. Hydrogen atoms always go on the outside so lets put the two Nitrogen atoms in the center. Diazene N2H2 or H2N2 CID 123195 - structure chemical names physical and chemical properties classification patents literature biological activities safety. In the Lewis structure for N2H2 there are a. Q9 6 PtsYT He Lewis Structure For N2H2.
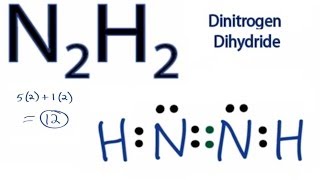 Source: youtube.com
Source: youtube.com
Also each Nitrogen atom carries one non-binding electron pair. Hydrogen atoms always go on the outside so lets put the two Nitrogen atoms in the center. Lewis structure for n2h2. Q9 6 PtsYT He Lewis Structure For N2H2. The molecule has a.
 Source: chegg.com
Source: chegg.com
N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. The Lewis structure of Diimide N₂H₂ is shown below. In the n 2 h 2 lewis structure the two nitrogen n atoms go in the center hydrogen always goes on the outside. In the N2H2 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Lewis Dot Structure For N2H2.
 Source: chegg.com
Source: chegg.com
Option-C each nitrogen has one nonbinding electron pair is the correct answer. Hydrogen atoms always go on the outside so lets put the two Nitrogen atoms in the center. 98 103 ratings Problem Details. Each hydrogen has one nonbonding electron pair lone pair FREE Expert Solution Show answer. Check out a sample QA here.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for n2h2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






