Lewis structure for n3
Lewis Structure For N3. N3- no need of hybridisation. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. The VSEPR model. Jul 11 2021 Because the Nitride ion N3- has an extra electron the negative sign denotes an extra electron we need to add that to the 5 valence electrons.
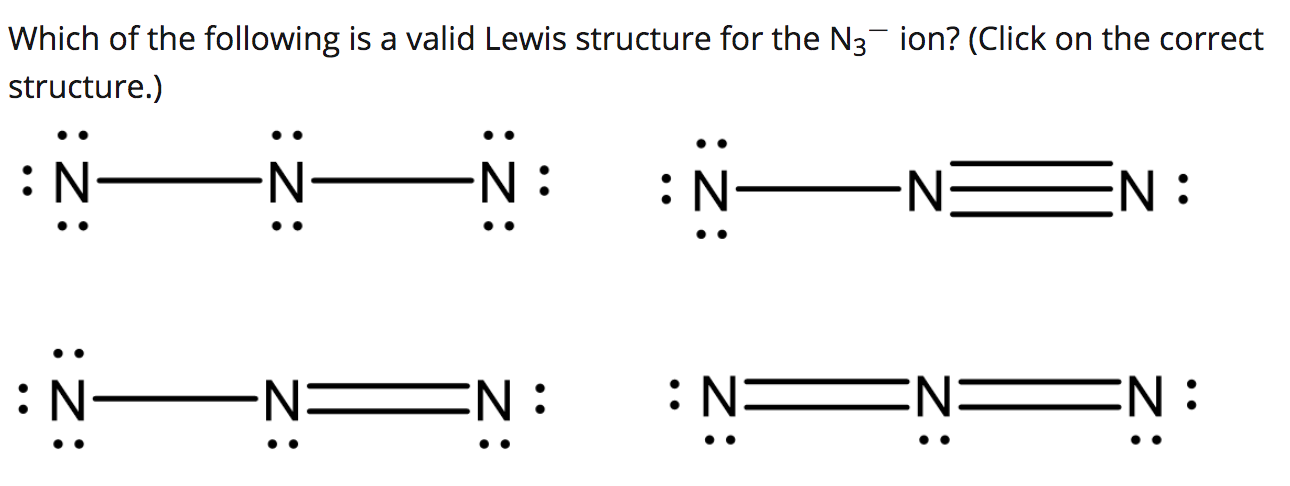 Which Of The Following Is A Valid Lewis Structure For Chegg Com From chegg.com
Which Of The Following Is A Valid Lewis Structure For Chegg Com From chegg.com
Lewis Dot Structure of N3- Azide Ion - YouTube. Azide Ion N3 - Lewis Dot Structure. The bond lengths for N1N2 and N4N5 are 110 Å and the. A step-by-step explanation of how to draw the N3- Lewis Dot Structure Azide ionFor the N3- structure use the periodic table to find the total number of va. Lewis structures are also called electron structures and they are diagrams which show possible bonds between the atoms that make up a molecule as well as any electron pairs which are unbonded. Well also explore polyatomic ions and how to draw Lewis dot structures for them.
With N 3- youll need to form two double bonds between the Nitrogen atoms to fill the octets and still use only the 34 valence electrons available for the molecule.
70 More Lewis Dot Structures. Add your answer and earn points. A step-by-step explanation of how to draw the N3- Lewis Dot Structure Azide ionFor the N3- structure use the periodic table to find the total number of va. Jul 11 2021 Because the Nitride ion N3- has an extra electron the negative sign denotes an extra electron we need to add that to the 5 valence electrons. Put brackets and a negative sign around the N 3- Lewis structure. Azide Ion N3 - Lewis Dot Structure - YouTube.
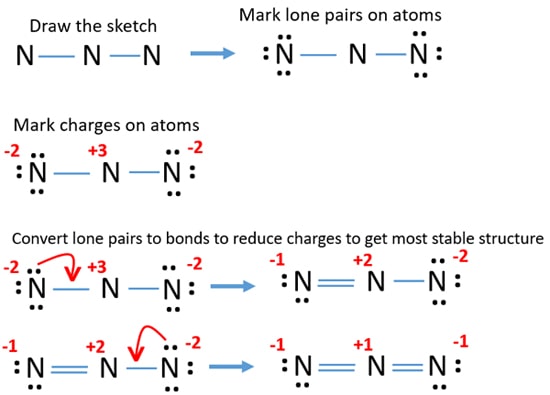 Source: chemistryscl.com
Source: chemistryscl.com
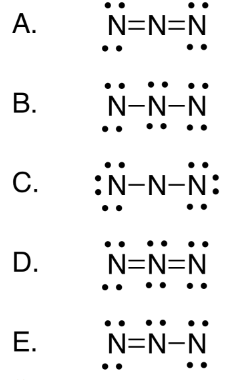
In lewis structure of N 3- ion contains two NN bonds. Lewis structure for n3-. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. Azide Ion N3 - Lewis Dot Structure - YouTube. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule.
 Source: youtube.com
Source: youtube.com
In lewis structure of N 3- ion contains two NN bonds. There are charges on all nitrogen atoms. Lewis structure for n3-. 1s2 2s2 2p3 Now that we know the number of valence electrons per element it is just a matter of drawing the electron dot configurationDraw The Lewis Structure For The Azide N3 Ion. Well also explore polyatomic ions and how to draw Lewis dot structures for them.
 Source: youtube.com
Source: youtube.com
1 The charges must be as least as possible if any. 1s2 2s2 2p3 Now that we know the number of valence electrons per element it is just a matter of drawing the electron dot configurationDraw The Lewis Structure For The Azide N3 Ion. N3- no need of hybridisation. Lewis structure for n3-. The VSEPR model.
 Source: youtube.com
Source: youtube.com
Find an answer to your question Lewis structure of n3- kapoorvriti923 kapoorvriti923 04052020 Chemistry Secondary School Lewis structure of n3- 1 See answer kapoorvriti923 is waiting for your help. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. 1 The charges must be as least as possible if any. There are a total of 16 valence electrons in the N 3- Lewis structure. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule.
 Source: brainly.com
Source: brainly.com
70 More Lewis Dot Structures. Azide Ion N3 - Lewis Dot Structure. Azide ion N 3- has only 3 nitrogen atoms. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. 2a The charges must be distributed over the molecu.
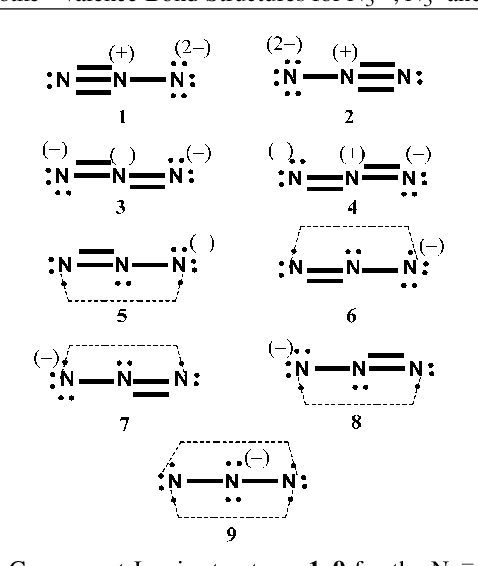 Source: novocom.top
Source: novocom.top
Put brackets and a negative sign around the N 3- Lewis structure. Azide Ion N3 - Lewis Dot Structure - YouTube. Well also explore polyatomic ions and how to draw Lewis dot structures for them. Put brackets and a negative sign around the N 3- Lewis structure. When considering the best stability for octet Lewis structures it is a matter of physics as we consider the stability of the electric charge.
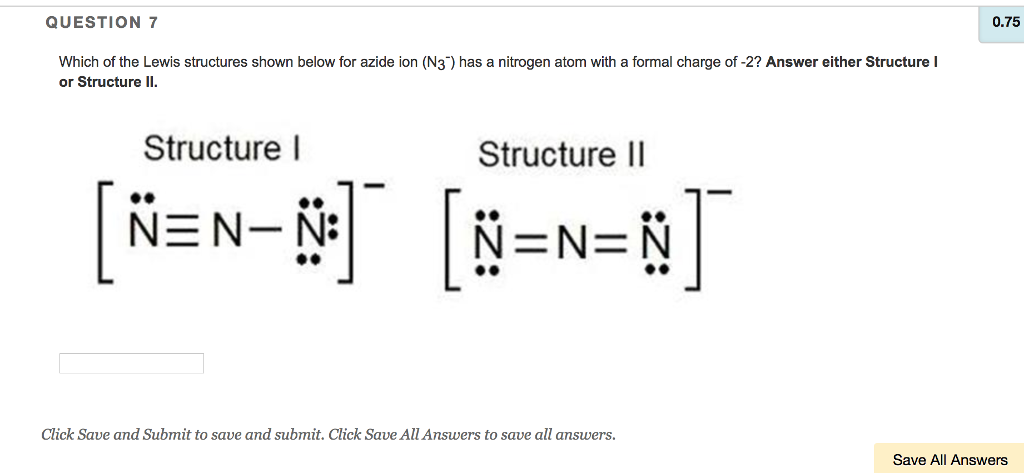 Source: chegg.com
Source: chegg.com
The bond lengths for N1N2 and N4N5 are 110 Å and the. 1s2 2s2 2p3 Now that we know the number of valence electrons per element it is just a matter of drawing the electron dot configurationDraw The Lewis Structure For The Azide N3 Ion. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. When considering the best stability for octet Lewis structures it is a matter of physics as we consider the stability of the electric charge. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence.
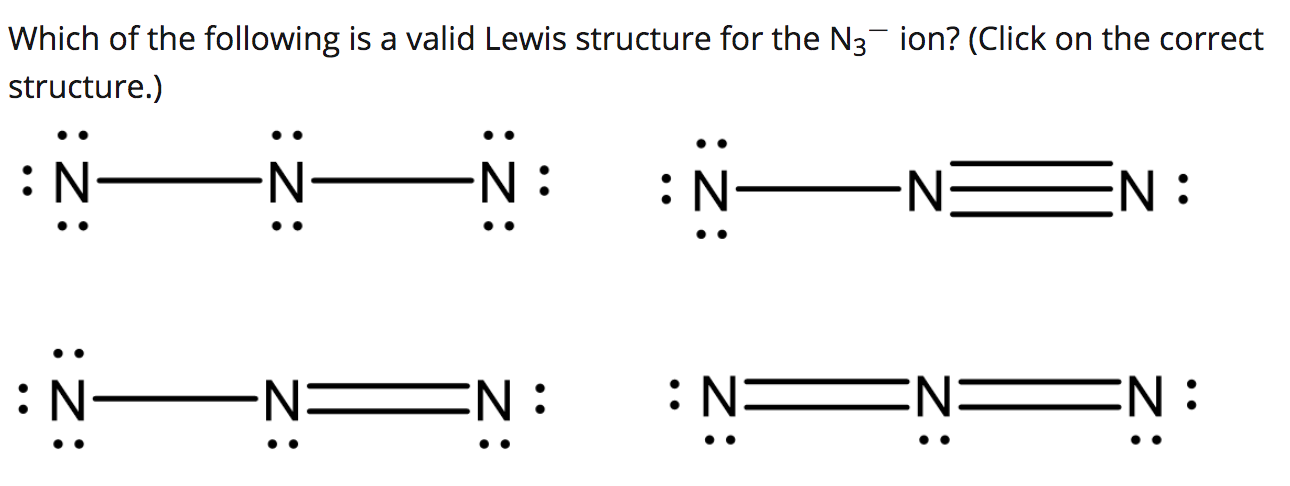 Source: chegg.com
Source: chegg.com
For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. Azide ion1- Azide1- Azide N3- Trinitrogen ion N3- Hydrazoic acid ion 1- azide group. Lewis Dot Structure of N3- Azide Ion - YouTube. Azide Ion N3 - Lewis Dot Structure - YouTube. There are a total of 16 valence electrons in the N 3- Lewis structure.
 Source: youtube.com
Source: youtube.com
In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. There are charges on all nitrogen atoms. In lewis structure of N 3- ion contains two NN bonds. Draw the Lewis structure for N3- and draw a seperate lewis structure for BH3. Azide Ion N3 - Lewis Dot Structure - YouTube.
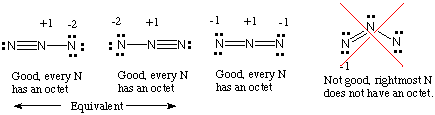 Source: socratic.org
Source: socratic.org
With N 3- youll need to form two double bonds between the Nitrogen atoms to fill the octets and still use only the 34 valence electrons available for the molecule. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. There are charges on all nitrogen atoms. With N 3- youll need to form two double bonds between the Nitrogen atoms to fill the octets and still use only the 34 valence electrons available for the molecule. Azide ion N 3- has only 3 nitrogen atoms.
 Source: study.com
Source: study.com
Trinitride1- trinitride 1- Azide negative ion. When considering the best stability for octet Lewis structures it is a matter of physics as we consider the stability of the electric charge. Put brackets and a negative sign around the N 3- Lewis structure. On a chemistry homework were asked to draw the Lewis structure of ceN3-. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule.
 Source: novocom.top
Source: novocom.top
Azide Ion N3 - Lewis Dot Structure. Lewis Dot Structure of N3- Azide Ion - YouTube. If playback doesnt begin shortly try restarting. A step-by-step explanation of how to draw the N3- Lewis Dot Structure Azide ionFor the N3- structure use the periodic table to find the total number of va. When considering the best stability for octet Lewis structures it is a matter of physics as we consider the stability of the electric charge.
 Source: novocom.top
Source: novocom.top
Each outside nitrogen atoms have two lone pairs and center nitrogen atom does not have lone pairs. Nitrogen ion N3- 14343-69-2. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. There are a total of 16 valence electrons in the N 3- Lewis structure.
 Source: youtube.com
Source: youtube.com
The VSEPR model. Lewis Dot Structure of N3- Azide Ion - YouTube. Nitrogen ion N3- 14343-69-2. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence. In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule.
 Source: chegg.com
Source: chegg.com
Lewis structures are also called electron structures and they are diagrams which show possible bonds between the atoms that make up a molecule as well as any electron pairs which are unbonded. The VSEPR model. With N 3- youll need to form two double bonds between the Nitrogen atoms to fill the octets and still use only the 34 valence electrons available for the molecule. N3- no need of hybridisation. 2a The charges must be distributed over the molecu.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for n3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






