Lewis structure for pcl3
Lewis Structure For Pcl3. A Lewis structure Set Al as the central atom with the Cl atoms directly attached to it. Lewis structure for PCL3 Other questions on the subject. When selecting small wares to use in the kitchen a manager must be sure the small wares are durable easy to clean safe and. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants.
 Question C9022 Socratic From socratic.org
Question C9022 Socratic From socratic.org
Lewis structure for pcl3 Phosphorus trichloride PCl3 contains three chlorine atoms and one phosphorus atoms. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants. You get the structure shown below. The lewis structure of PCl3 can be explained as follows. Also there is a lone pair on phosphorus atom. Which in turn enjoy.
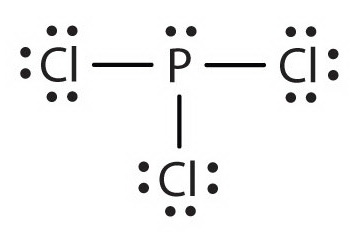
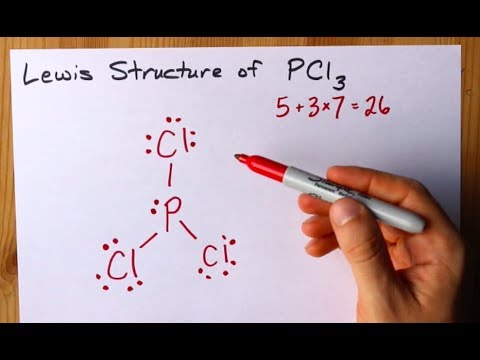
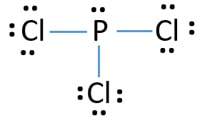
Phosphorus Trichloride PCl3 has a total of 26 valence electrons.
In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. PCl3 Lewis Structure The lewis structure of PCl3 can be explained as follows. B Geometry There are six bond pairs and no lone pairs about the Al. This would be see double bond and h lone pair each and. PCl 3 is important indirectly as a precursor to PCl 5 POCl 3 and PSCl 3. The A represents the central atom the phosphorus each.
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
PCl3 lewis structure In this lewis. Also there is a lone pair on phosphorus atom. All of the following Lewis structures of nitrogen oxides are possible EXCEPT. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants. For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table.
 Source: youtube.com
Source: youtube.com
You know what for D s F two to impairs on sulfur. The A represents the central atom the phosphorus each. You know what for D s F two to impairs on sulfur. PCl 3 is important indirectly as a precursor to PCl 5 POCl 3 and PSCl 3. Lewis structure for PCL3.
 Source: techiescientist.com
Source: techiescientist.com
The A represents the central atom the phosphorus each. Which in turn enjoy. Phosphorous chloride Lewis Structure. Lewis structure for PCL3 Other questions on the subject. B Geometry There are six bond pairs and no lone pairs about the Al.
 Source: socratic.org
Source: socratic.org
In a Hoechst continuous process molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. Here in this post we. Phosphorus Trichloride PCl3 has a total of 26 valence electrons. In a Hoechst continuous process molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. Correct answer to the question.
 Source: quora.com
Source: quora.com
Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants. Layers of rock containing fossils like the layers illustrated here are most likely composed of rocks. SF two for E h 2 cc h two f h and N h yeah and double bond and H. The straw Lewis structures for each of the following molecules for a H two is a little structure h each for be hbr. Each home pairs here Frenchy H two c n h.
 Source: novocom.top
Source: novocom.top
B Geometry There are six bond pairs and no lone pairs about the Al. The A represents the central atom the phosphorus each. In this tutorial we will learn how to draw the lewis structure of PCl3 with all theories. In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. You get the structure shown below.
 Source: youtube.com
Source: youtube.com
Each chlorine has 63 lone pairs. PCl 3 Phosphorus Trichloride Lewis Structure Phosphorus trichloride PCl 3 contains three chlorine atoms and one phosphorus atoms. Lewis structure for pcl3 Phosphorus trichloride PCl3 contains three chlorine atoms and one phosphorus atoms. This would be see double bond and h lone pair each and. Correct answer to the question.
 Source: youtube.com
Source: youtube.com
SF two for E h 2 cc h two f h and N h yeah and double bond and H. Also there is a lone pair on phosphorus atom. Each chlorine has 63 lone pairs. Lewis structure for PCL3. You know what for D s F two to impairs on sulfur.
 Source: youtube.com
Source: youtube.com
Electrons available Al 6Cl 3- 3 66 3 6 36 42 Arrange these electrons to give every atom an octet. PCl 3 is similar to PBr 3 and PF 3. You get the structure shown below. PCl3 lewis structure In this lewis. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants.
 Source: byjus.com
Source: byjus.com
2 Get Other questions on the subject. In PCl 3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. Also there is a lone pair on phosphorus atom. Which in turn enjoy. A Lewis structure Set Al as the central atom with the Cl atoms directly attached to it.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table. Also there is a lone pair on phosphorus atom. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants. Draw the Lewis structure for HCN which has a triple bond. All of the following Lewis structures of nitrogen oxides are possible EXCEPT.
 Source: chemistryscl.com
Source: chemistryscl.com
The phosphorus trichloride chemical formula is PCl3. Carbon c is least electronegative atom and goes in center o. Phosphorus Trichloride PCl3 has a total of 26 valence electrons. Here in this post we. In a Hoechst continuous process molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride.
 Source: study.com
Source: study.com
For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table. For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table. All of the following Lewis structures of nitrogen oxides are possible EXCEPT. Phosphorous chloride Lewis Structure. How do you know when you need to add one or more multiple bonds to complete a Lewis structure.
 Source: techiescientist.com
Source: techiescientist.com
These chlorines want to satisfy their oxide requirement and that is why the geometry for PCL3 is called Trigonal Pyramidal. PCl 3 Phosphorus Trichloride Lewis Structure Phosphorus trichloride PCl 3 contains three chlorine atoms and one phosphorus atoms. The straw Lewis structures for each of the following molecules for a H two is a little structure h each for be hbr. The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. Drawing PCl3 Lewis Structure is very easy to by using the following method.
Source: quora.com
How to Draw a Lewis Structure for PCl3. Electrons available Al 6Cl 3- 3 66 3 6 36 42 Arrange these electrons to give every atom an octet. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants. To draw the lewis structure first of all we need to sum up the valence electrons of all the atoms. Now lets move on to the lewis structure of PCl3.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure for pcl3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.