Lewis structure for sf6
Lewis Structure For Sf6. SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom. Posted on June 24 2015. The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees.
 Sf6 Lewis And 3 D Structures Dr Sundin Uw Platteville From people.uwplatt.edu
Sf6 Lewis And 3 D Structures Dr Sundin Uw Platteville From people.uwplatt.edu
In the Lewis structure for SF6 the central sulfur atom shares _____ electrons. Es ist unter Normalbedingungen ein farb- und geruchloses ungiftiges und nicht brennbares Gas das sich ähnlich wie Stickstoff äußerst reaktionsträge verhält. What is the Lewis structure of SF6. Molecular Orbitals in SF6 Molecular Orbitals in SF6. There are a total of 48 valence electrons in the Lewis structure for SF6. SF6 Lewis and 3-D Structures - Dr.
None of the above because SF6 is an ionic compound.
Posted on June 24 2015. How to draw the lewis structure of SF6 - YouTube. Lewis Structure For Sf6 Free PDF eBooks. Asked Jun 19 2017 in Chemistry by JockaJoc. Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. Alternatively a dot method can be used to draw the lewis structure.
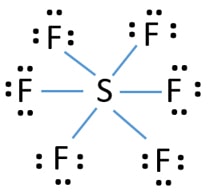 Source: chemistryscl.com
Source: chemistryscl.com
A step-by-step explanation of how to draw the SF6 Lewis Structure Sulfur Hexafluroide For the SF6 Lewis structure we first count the valence electrons for the SF6 molecule using the periodic table. None of the above because SF6 is an ionic compound. I also go over the hybridization shape and bond angles. Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. None of the above because SF6 is an ionic compound.
 Source: biochemhelp.com
Source: biochemhelp.com
There are a total of 48 valence electrons in the Lewis structure for SF6. Put sulfur in the center and five fluorine atoms on the sides. How to draw the lewis structure of SF6. Molecular Orbitals in SF6 Molecular Orbitals in SF6. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom.
 Source: youtube.com
Source: youtube.com
Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. Lewis Structure For Sf6 Free PDF eBooks. Expanded octet lewis structure. A step-by-step explanation of how to draw the SF6 Lewis Structure Sulfur Hexafluroide For the SF6 Lewis structure we first count the valence electrons for the SF6 molecule using the periodic table. I quickly take you through how to draw the Lewis Structure of SF6 Sulfur HexaFluoride.
 Source: clutchprep.com
Source: clutchprep.com
Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. Answered Jun 19 2017 by mi_flux. The Lewis structure of SF6 describes six pairs of electrons as bond pairs despite the availability of only four valence orbitals. In order to calculate the formal charges for SF6 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding elec. How to Draw the Lewis Structure for SF6.
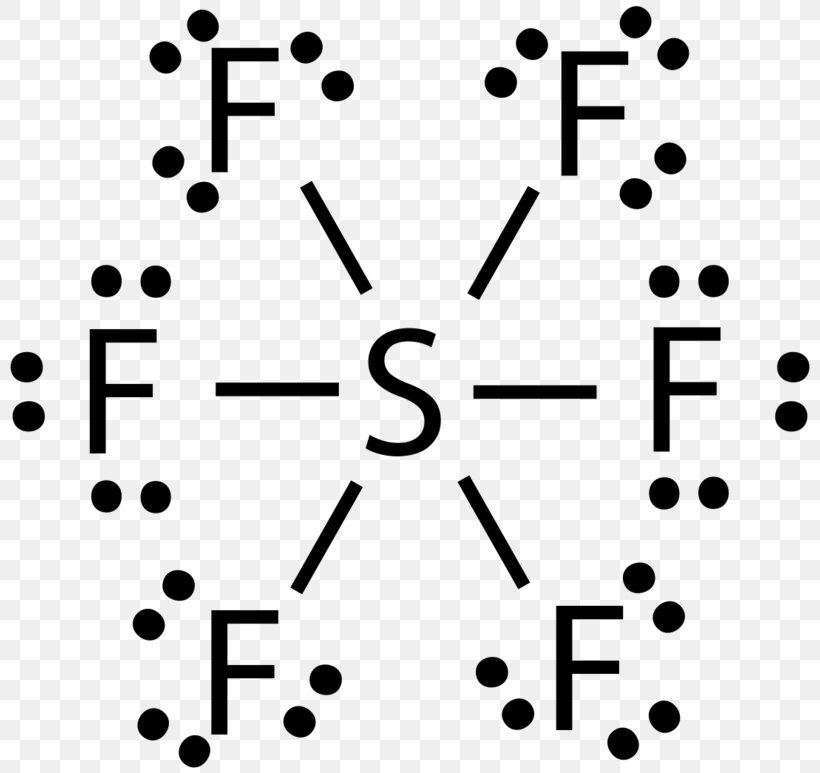 Source: favpng.com
Source: favpng.com
Expanded octet lewis structure. Expanded octet lewis structure. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom. How to Draw the Lewis Structure for SF6. Calculate the total valence electrons in the molecule.
 Source: youtube.com
Source: youtube.com
Alternatively a dot method can be used to draw the lewis structure. SF 6 Sulfur hexafluoride Lewis Structure. Once we know how many valence electrons there are in SF6 we can distribute them. SF6 Lewis and 3-D Structures - Dr. How to Draw the Lewis Structure for SF6.
 Source: techiescientist.com
Source: techiescientist.com
Alternatively a dot method can be used to draw the lewis structure. Posted on June 24 2015. None of the above because SF6 is an ionic compound. Calculate the total valence electrons in the molecule. What is the Lewis structure of SF6.
 Source: techiescientist.com
Source: techiescientist.com
There are a total of 48 valence electrons in the Lewis structure for SF6. There are a total of 48 valence electrons in the Lewis structure for SF6. What is the Lewis structure of SF6. Expanded octet lewis structure. Chapter 3 - Molecular Shape and Structure The terminal atoms are identical.

Put sulfur in the center and five fluorine atoms on the sides. SF6 is a colorless and odorless gas that is non-combustible and non-flammable in nature. In the Lewis structure for SF6 the central sulfur atom shares _____ electrons. How to Draw the Lewis Structure for. Expanded octet lewis structure.
 Source: youtube.com
Source: youtube.com
Lewis dot structure has 6 sigma bonds and rests lone pairs on fluorine. The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees. How to draw the lewis structure of SF6. What is the Lewis structure of SF6. Molecular Orbitals in SF6 Molecular Orbitals in SF6.
 Source: people.uwplatt.edu
Source: people.uwplatt.edu
Answered Jun 19 2017 by mi_flux. The hybridization of Sulphur in this molecule is sp3d2 with the bond angles of 90 degrees. Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. Lewis structure of SF 6 is given below. To do so we first need to do the following steps.
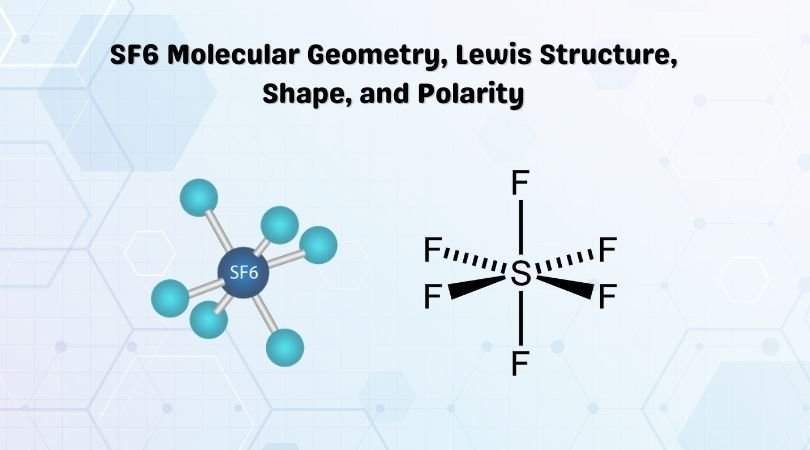 Source: geometryofmolecules.com
Source: geometryofmolecules.com
The hybridization of SF6 is sp3d2. To do so we first need to do the following steps. There are a total of 48 valence electrons in the Lewis structure for SF6. Expanded octet lewis structure. A step-by-step explanation of how to draw the SF6 Lewis Structure Sulfur Hexafluroide For the SF6 Lewis structure we first count the valence electrons for the SF6 molecule using the periodic table.
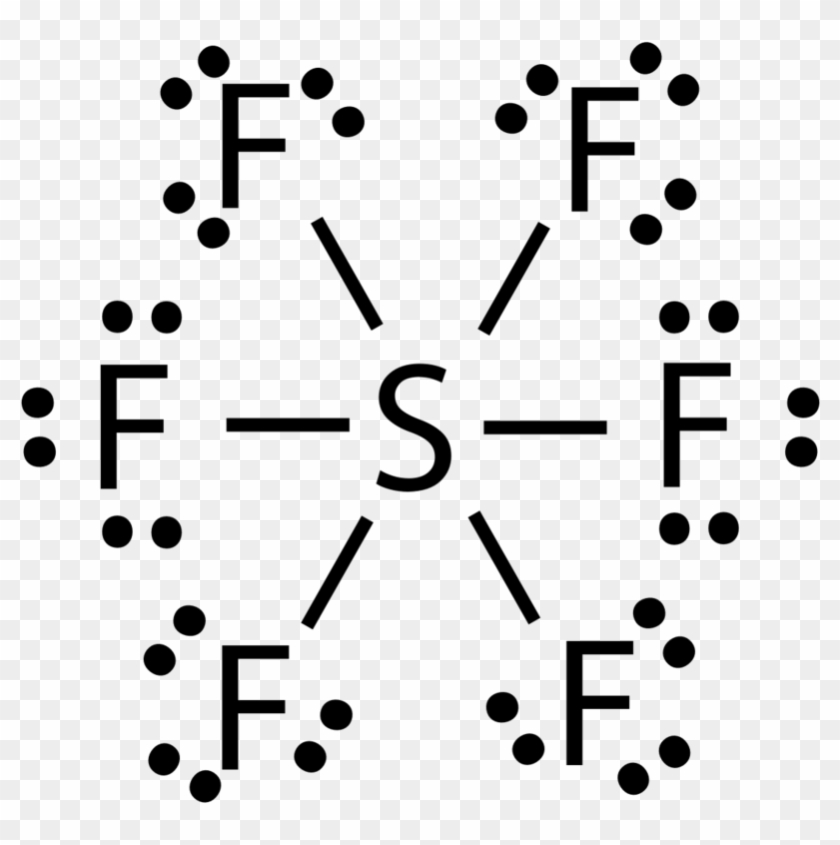 Source: clipartmax.com
Source: clipartmax.com
Lewis Structure For Sf6 Free PDF eBooks. Lewis dot structure of SF 6. In this tutorial we will learn how to draw the lewis structure of SF. SF 6 Sulfur hexafluoride molecule contains one sulfur atom and six fluorine atoms. To summarize this article we can say that in the Lewis dot structure of SF6 all the valence electrons are used up which results in forming six single bonds between S-F with no lone pairs of electrons.
 Source: novocom.top
Source: novocom.top
Asked Jun 19 2017 in Chemistry by JockaJoc. Lewis dot structure of SF 6. Determine the central atom in this molecule. Lewis structure of SF 6 is given below. Asked Jun 19 2017 in Chemistry by JockaJoc.
 Source: people.uwplatt.edu
Source: people.uwplatt.edu
ReadDownload File Report Abuse. A step-by-step explanation of how to draw the SiF62- Lewis Dot StructureFor the SiF6 2- Lewis structure calculate the total number of valence electrons for. Once we know how many valence electrons there are in SF6 we can distribute them. The central atom here is sulfur bonded with 6 fluorine atoms. SF 6 Sulfur hexafluoride Lewis Structure.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis structure for sf6 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





