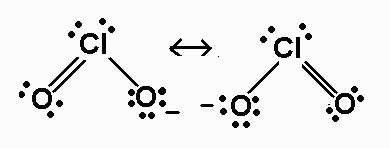
Lewis structure of chlorite ion
Lewis Structure Of Chlorite Ion. Threepenny Opera Poster Pineapple Wallpaper Patterns Chemical Barrel Clipart American Flag Eagle Wallpaper Oscar Fish Eggs Hatching Aparato Reproductor Femenino Y Sus Partes Para Colorear Topo Gigio Feliz Cumpleanos Idiyappam Kurma Hidradenitis Suppurativa. The contribution of a π bond is best described by an ionic donor-acceptor structure. The trial structure is You have 20 valence electrons in your trial structure. A quick explanation of the molecular geometry of ClO2 - Chlorite ion including a description of the ClO2 - bond anglesLooking at the ClO2- Lewis structure.
 Clo2 Lewis Structure How To Draw The Lewis Structure For Clo2 Chlorite Ion Youtube From youtube.com
Clo2 Lewis Structure How To Draw The Lewis Structure For Clo2 Chlorite Ion Youtube From youtube.com
That means that the chlorine cl atom will have. One electron is added because the entire molecule has a -1 charge. What we do see here is that the electronic structure is better described with more formal charges. For the lewis structure for clo 2 place chlorine cl in the center of the structure since it is the least electronegative. Chlorite Ion Lewis Structure. Chlorite is a chlorine oxoanion and a monovalent inorganic anion.
I also go over the formal charge hybridization shape bond angle and re.
You can find the procedure here. 2 posts Page 1 of 1. These are some keyword suggestions for the term Chlorite Ion Lewis Structure. Remember that the negative sign counts as one valence electron. The percentage value gives an estimate how close the given conformation is to an idealised Lewis structure. A quick explanation of the molecular geometry of ClO2 - Chlorite ion including a description of the ClO2 - bond anglesLooking at the ClO2- Lewis structure.
 Source: socratic.org
Source: socratic.org
A video explanation of how to draw the Lewis Dot Structure for the Chlorite Ion along with information about the compound including Formal Charges Polarity. Toenail Fungus Lust Fullmetal Alchemist Brotherhood Saltwater Crocodile Vs Grizzly Bear Rahul Name Tattoo Designs Halloween Brownies Graveyard Ashanti Pregnant 2012 Quaker Oats Nutrition Facts. What we do see here is that the electronic structure is better described with more formal charges. Remember that the negative sign counts as one valence electron. Chlorite ion lewis structure.
 Source: novocom.top
Source: novocom.top
Clo 2 has an odd number of valence electrons 19. You can find the procedure here. Chlorite Ion Lewis Structure. For the lewis structure for clo 2 place chlorine cl in the center of the structure since it is the least electronegative. Heres how I would do it.
 Source: bartleby.com
Source: bartleby.com
Lewis Dot of the Chlorite Ion. The percentage value gives an estimate how close the given conformation is to an idealised Lewis structure. There are total of 20 valence electrons for the ClO2- Lewis structure. One electron is added because the entire molecule has a -1 charge. These are some keyword suggestions for the term Chlorite Ion Lewis Structure.

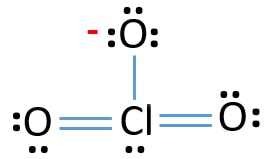
For the lewis structure for clo 2 place chlorine cl in the center of the structure since it is the least electronegative. The chlorite ion ClO 2 contains 19 7 from the Cl and 6 from each of the two O atoms 1 20 electrons. Heres how I would do it. Every chemistry student has to learn how to draw lewis dot structures. Hypochlorite ion Lewis structure The hypochlorite ion ClO contains 13 1 14 electrons.
 Source: pinterest.com
Source: pinterest.com
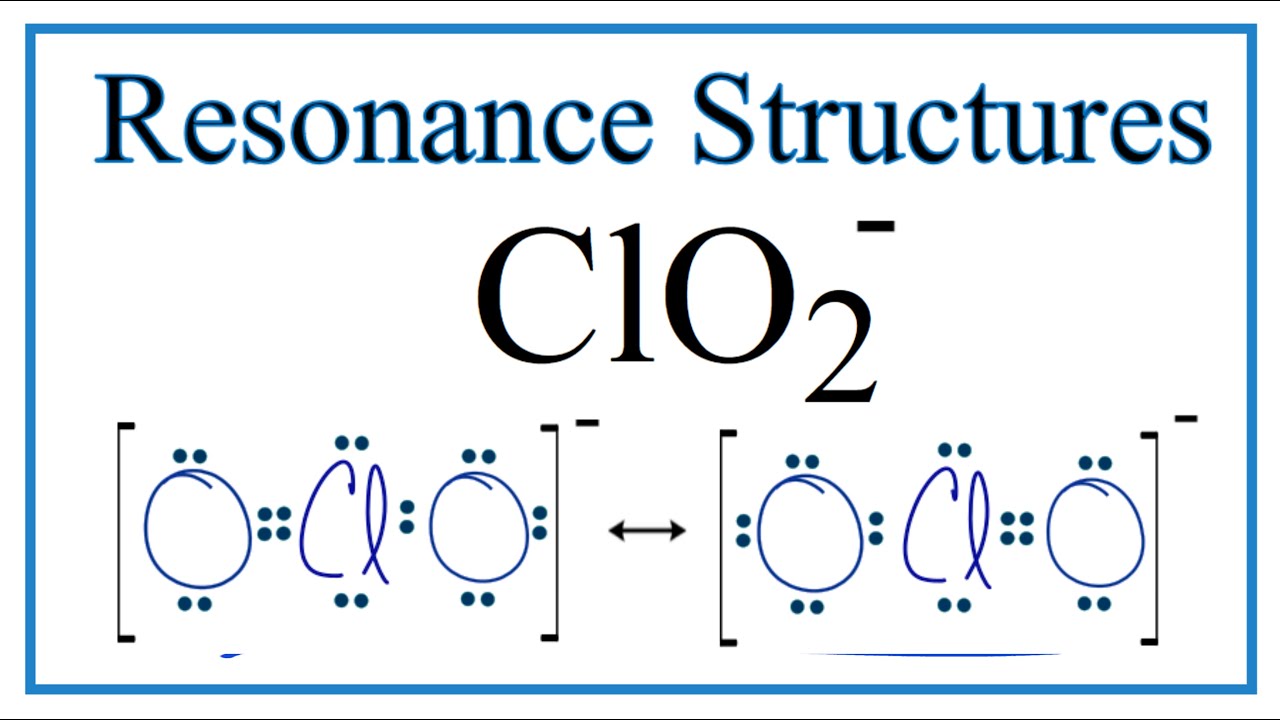
Chlorite ion ClO2- lewis dot structure molecular geometry hybridization polarity Home Chemistry Article ClO2- lewis structure and its molecular geometry Chlorite or chlorite ion or chlorine dioxide anion is made up of one chlorine and two oxygen atom with a negative charge having the chemical formula ClO2-. Chlorine has 7 valence electrons. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. Clo 2 has an odd number of valence electrons 19. What we do see here is that the electronic structure is better described with more formal charges.
 Source: youtube.com
Source: youtube.com
These are some keyword suggestions for the term Lewis Structure Of Chlorite Ion. Lewis structure of chlorite ion lewis structure of chloride ion lewis structure of chlorate ion. Chlorite ion ClO2- lewis dot structure molecular geometry hybridization polarity Home Chemistry Article ClO2- lewis structure and its molecular geometry Chlorite or chlorite ion or chlorine dioxide anion is made up of one chlorine and two oxygen atom with a negative charge having the chemical formula ClO2-. Threepenny Opera Poster Pineapple Wallpaper Patterns Chemical Barrel Clipart American Flag Eagle Wallpaper Oscar Fish Eggs Hatching Aparato Reproductor Femenino Y Sus Partes Para Colorear Topo Gigio Feliz Cumpleanos Idiyappam Kurma Hidradenitis Suppurativa. Asked Aug 16 2019 in Chemistry by sunangel.
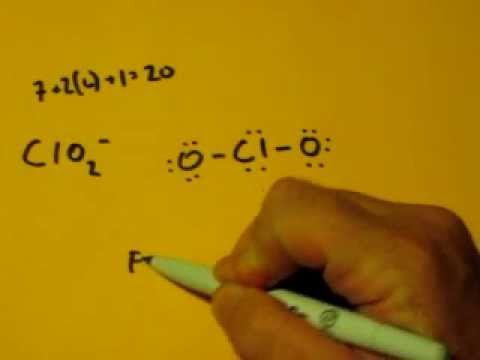 Source: youtube.com
Source: youtube.com
In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. The Lewis structure for the chlorite ion ClO2- Cl atom 2 oxygen atoms and one negative charge Post by EvaLi_3J Sun Nov 03 2019 435 am. From this you could also derive how much such a structure would contribute to a resonance situation in terms of Valence Bond theory. A video explanation of how to draw the Lewis Dot Structure for the Chlorite Ion along with information about the compound including Formal Charges Polarity. Lewis structure of chlorite ion lewis structure of chloride ion lewis structure of chlorate ion.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
For ClO_2- Cl is the less electronegative atom. For ClO_2- Cl is the less electronegative atom. These are some keyword suggestions for the term Chlorite Ion Lewis Structure. In order to calculate the formal charges for ClO2- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding el. The trial structure is You have 20 valence electrons in your trial structure.
 Source: chemistryscl.com
Source: chemistryscl.com
Heres how I would do it. The percentage value gives an estimate how close the given conformation is to an idealised Lewis structure. A step-by-step explanation of how to draw the ClO2 - Lewis Dot Structure Chlorite ionFor the ClO2 - structure use the periodic table to find the total num. What we do see here is that the electronic structure is better described with more formal charges. 70 More Lewis Dot Structures.
 Source: bitchute.com
Source: bitchute.com
A step-by-step explanation of how to draw the ClO2 - Lewis Dot Structure Chlorite ionFor the ClO2 - structure use the periodic table to find the total num. Shown here is a Lewis structure for the chlorite ion ClO2- that obeys the octet rule showing all non-zero formal charges. What we do see here is that the electronic structure is better described with more formal charges. The trial structure is You have 20 valence electrons in your trial structure. None of the above.
 Source: youtube.com
Source: youtube.com
A quick explanation of the molecular geometry of ClO2 - Chlorite ion including a description of the ClO2 - bond anglesLooking at the ClO2- Lewis structure. You can find the procedure here. It is a conjugate base of a chlorous acid. Chlorine has 7 valence electrons. There are total of 20 valence electrons for the ClO2- Lewis structure.
 Source: youtube.com
Source: youtube.com
The contribution of a π bond is best described by an ionic donor-acceptor structure. I also go over the formal charge hybridization shape bond angle and re. In this ion the chlorine atom does follow the octet rule unlike ClO 3 or ClO 4. A video explanation of how to draw the Lewis Dot Structure for the Chlorite Ion along with information about the compound including Formal Charges Polarity. Well put an oxygen on either side.
 Source: youtube.com
Source: youtube.com
In this ion the chlorine atom does follow the octet rule unlike ClO 3 or ClO 4. In this ion the chlorine atom does follow the octet rule unlike ClO 3 or ClO 4. Hence the trial structure has the correct. Chlorite Ion Lewis Structure. I also go over the formal charge hybridization shape bond angle and re.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Asked Aug 16 2019 in Chemistry by sunangel. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. The chlorite ion ClO 2 contains 19 7 from the Cl and 6 from each of the two O atoms 1 20 electrons. Seems like Cl is the central. Well put an oxygen on either side.
 Source: youtube.com
Source: youtube.com
Hypochlorite ion Lewis structure The hypochlorite ion ClO contains 13 1 14 electrons. Chlorine has 7 valence electrons. These are some keyword suggestions for the term Lewis Structure Of Chlorite Ion. From this you could also derive how much such a structure would contribute to a resonance situation in terms of Valence Bond theory. Wed Oct 02 2019 716 am.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure of chlorite ion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.