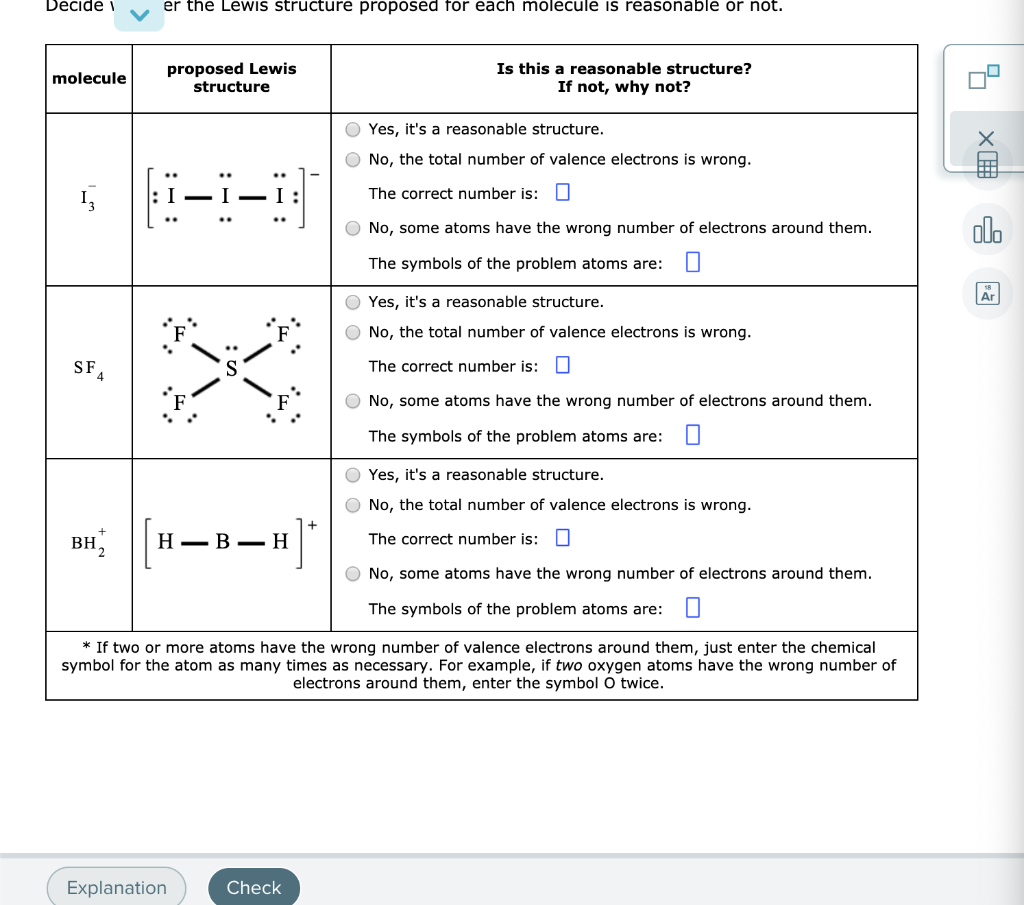
Lewis structure of i3
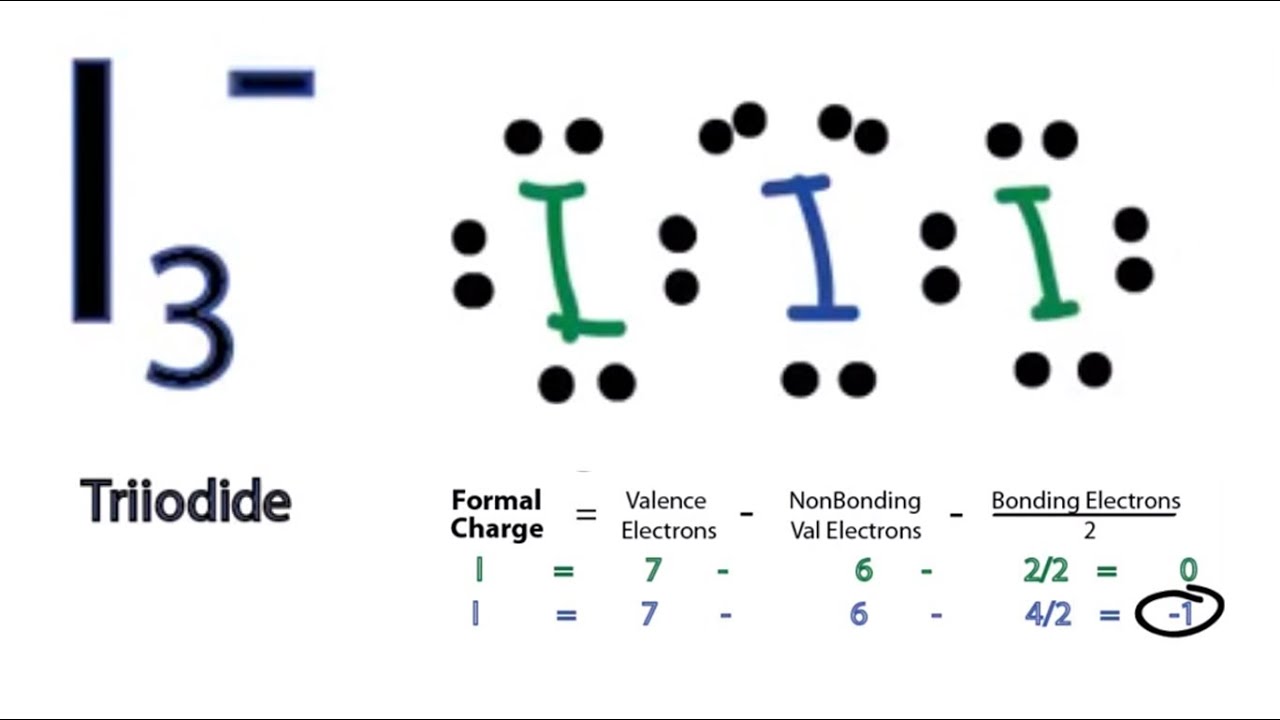
Lewis Structure Of I3. While there are 3 Iodine atoms one of the atoms has a negative charge which further gives 2 bond pairs and 3 lone pairs of electrons. Due to this one extra electron there 3 lone pairs of electrons and 2 bond pairs making its steric number 5. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. There three molecules of iodine in this molecule and hence the name of the compound are Triodide.
 Although I3 Is Known F3 Is Not Draw The Clutch Prep From clutchprep.com
Although I3 Is Known F3 Is Not Draw The Clutch Prep From clutchprep.com
There are three Iodine atoms out of which one has an extra negative charge. The brackets are placed around the Lewis structure because the diagram is of a polyatomic ion. It also discusses the molecular geometry bond angle hybridization and form. Knowing the i3 Lewis structure and understanding its physical properties hybridization and molecule shape is critical. For I3- well end up with 6 additional valence electrons after filling the octets. Fri Sep 28 2018 718 am.
It is formed by combining aqueous solutions of iodide salts and iodine.
It is formed by combining aqueous solutions of iodide salts and iodine. In closing remarks I3 Lewis Structure- may be a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization to sum up this complete article. I2 I- - I3-. Include all lone pairs of electrons. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. It is characterized as a toxic colorless gas that is heavier than air with a disagreeable odor and exposure occurs by inhalation ingestion or contact.
 Source: study.com
Source: study.com
It is formed by combining aqueous solutions of iodide salts and iodine. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. Follow Report by Prakharmishra1738 07112019 Log in to add a. I2 I- - I3-. Te Jung Yang 4K Posts.
 Source: youtube.com
Source: youtube.com
It is formed by combining aqueous solutions of iodide salts and iodine. There are three Iodine atoms out of which one has an extra negative charge. It is characterized as a toxic colorless gas that is heavier than air with a disagreeable odor and exposure occurs by inhalation ingestion or contact. Structure My Deal tools are complete youre ready to visit BMW of Reading. I2 I- - I3-.
 Source: chegg.com
Source: chegg.com
The 3 lone pairs will repel each other and take up the equatorial positions. Fri Sep 28 2018 718 am. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. 70 More Lewis Dot Structures. 3 likes 1 talking about this.
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
For understanding the Lewis structure of. I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. Ask for details. A step-by-step explanation of how to draw the I3 - Lewis Dot Structure Triiodide IonFor the I3 - structure use the periodic table to find the total number. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The shape of the molecule I3- is Linear. This chemistry video tutorial explains how to draw the lewis structure of I3-. I dont understand how to make the I3- lewis structure. Lewis structure of I3-Moderators. What is the Lewis structure of I3.
 Source: youtube.com
Source: youtube.com
It is characterized as a toxic colorless gas that is heavier than air with a disagreeable odor and exposure occurs by inhalation ingestion or contact. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. It is important to know the Lewis structure of a molecule to understand its physical properties hybridization and shape of the molecule. The 3 lone pairs will repel each other and take up the equatorial positions. I quickly take you through how to draw the Lewis Structure of I3- TriIodide Ion.
 Source: techiescientist.com
Source: techiescientist.com
I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram. It also discusses the molecular geometry bond angle hybridization and form. While there are 3 Iodine atoms one of the atoms has a negative charge which further gives 2 bond pairs and 3 lone pairs of electrons. It is important to know the Lewis structure of a molecule to understand its physical properties hybridization and shape of the molecule. What is the Lewis structure of I3.
 Source: clutchprep.com
Source: clutchprep.com
I2 I- I3- This is the exergonic equilibrium leading to the formation of the ion where a. This chemistry video tutorial explains how to draw the lewis structure of I3-. For understanding the Lewis structure of. In closing remarks I3 Lewis Structure- may be a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization to sum up this complete article. What is the Lewis structure of I3.
 Source: techiescientist.com
Source: techiescientist.com
This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. In closing remarks I3 Lewis Structure- may be a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization to sum up this complete article. Once we know how many valence electrons there are in I3- we can distribute them around the central atom and attempt to fill the outer shells of each atom. Due to this one extra electron there 3 lone pairs of electrons and 2 bond pairs making its steric number 5. 5 points Draw the Lewis dot structure of I3.
 Source: youtube.com
Source: youtube.com
While there are 3 Iodine atoms one of the atoms has a negative charge which further gives 2 bond pairs and 3 lone pairs of electrons. The brackets are placed around the Lewis structure because the diagram is of a polyatomic ion. However it is hard. I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. Ask for details.
 Source: youtube.com
Source: youtube.com
However it is hard. In closing remarks I3 Lewis Structure- may be a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization to sum up this complete article. Triiodide is observed to be a red colour in solution. We are getting to undergo. This chemistry video tutorial explains how to draw the lewis structure of I3-.
 Source: clutchprep.com
Source: clutchprep.com
For understanding the Lewis structure of. I also go over hybridization shape and bond angle. Te Jung Yang 4K Posts. Due to this one extra electron there 3 lone pairs of electrons and 2 bond pairs making its steric number 5. I quickly take you through how to draw the Lewis Structure of I3- TriIodide Ion.
Source: quora.com
I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I dont understand how to make the I3- lewis structure. Today we are going to go through the Lewis structure of I3- or also know as Triodide ion as it has a negative charge on it. What is the Lewis structure of I3. I3 Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1.
 Source: brainly.in
Source: brainly.in
For understanding the Lewis structure of. It is characterized as a toxic colorless gas that is heavier than air with a disagreeable odor and exposure occurs by inhalation ingestion or contact. I dont understand how to make the I3- lewis structure. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. In closing remarks I3 Lewis Structure- may be a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization to sum up this complete article.
 Source: byjus.com
Source: byjus.com
This chemistry video tutorial explains how to draw the lewis structure of I3-. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. In closing remarks I3 Lewis Structure- may be a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization to sum up this complete article. There are three Iodine atoms out of which one has an extra negative charge. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure of i3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






