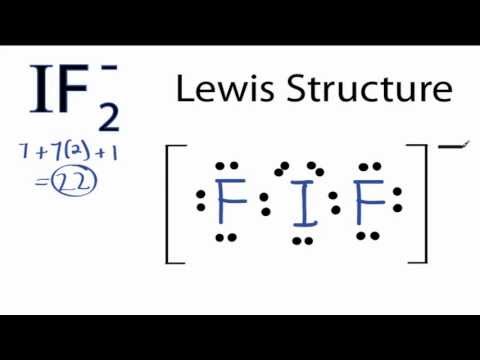
Lewis structure of if2
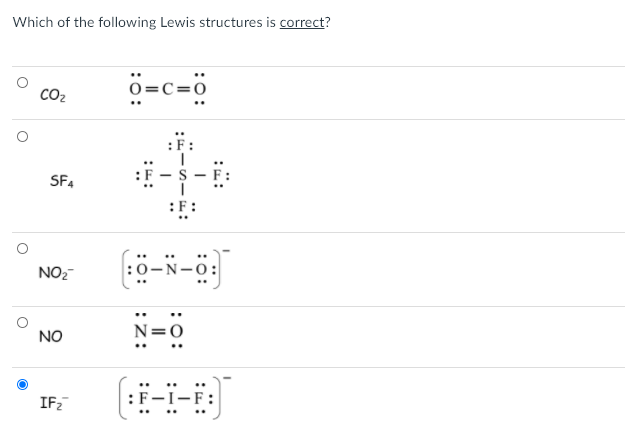
Lewis Structure Of If2. C What value do you expect for the F-I-F angle. Tetrahedral as it has four group of. A step-by-step explanation of how to draw the IF2- Lewis Structure. The Lewis structures for these molecules or ions are given below.
 If2 Lewis Structure How To Discuss From howtodiscuss.com
If2 Lewis Structure How To Discuss From howtodiscuss.com
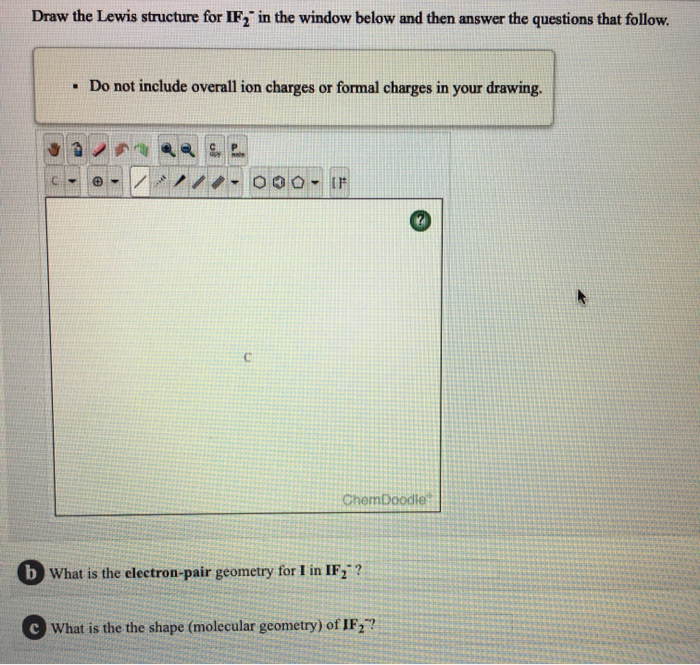
Share on Tumblr. In the IF 2- Lewis structure Iodine I is the least electronegative atom and goes in the center of the Lewis structure. Can O3 AsH3 and H2Se act as a lewis acid. The sulfur atom has six valence electrons and each fluorine has seven valence electrons so the Lewis electron structure is. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model. 4 Question 7 6 Marks a Draw the Lewis structure of IF2.
4 Question 7 6 Marks a Draw the Lewis structure of IF2.
A step-by-step explanation of how to draw the IBr4 - Lewis Dot StructureFor the IBr4 - structure use the periodic table to find the total number of valence. Get 15 discount on your first 3 orders with us Use the following coupon FIRST15 Order Now. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model. Bookkeeping 101 Solution. Draw the Lewis structure of NH2 Draw the Lewis structure of CH33O Labelor unlabel the sp-hybridized atoms sp2-hybridized atoms and sp3-hybridized atoms. Done on a Dell Dimension laptop computer with a Wacom digital tablet Bamboo.
 Source: oneclass.com
Source: oneclass.com
The sulfur atom has six valence electrons and each fluorine has seven valence electrons so the Lewis electron structure is. Drawing the Lewis Structure for IF 5. Fluoroethyne C2HF CID 32759 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Can O3 AsH3 and H2Se act as a lewis acid. The Lewis structures for these molecules or ions are given below.
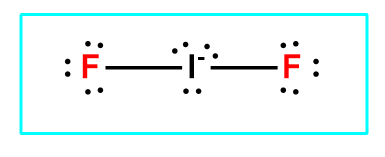 Source: clutchprep.com
Source: clutchprep.com
B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model. Which of the following is not true about the structure of the alveoli A. In our company we have structured levels of accounting services solutions which we refer to as. Write the Lewis. The IF 2- Lewis structure youll need to put more than eight valence electrons on the Iodine atome.
 Source: youtube.com
Source: youtube.com
What is the lewis structure for N-bromosuccinimide. The Lewis structures for these molecules or ions are given below. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model. A step-by-step explanation of how to draw the IBr4 - Lewis Dot StructureFor the IBr4 - structure use the periodic table to find the total number of valence. The Lewis structure of shows that the central phosphorus atom has _____ nonbonding and _____ bonding electron pairs.
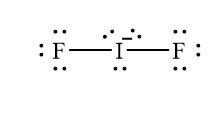 Source: study.com
Source: study.com
4 Question 7 6 Marks a Draw the Lewis structure of IF2. Bookkeeping 101 Solution. The IF 2- Lewis structure youll need to put more than eight valence electrons on the Iodine atome. NH3 electron geometry is. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model.
 Source: clutchprep.com
Source: clutchprep.com
Done on a Dell Dimension laptop computer with a Wacom digital tablet Bamboo. See the Big List of Lewis Structures. 4178 results page 5 chem. A step-by-step explanation of how to draw the IF2- Lewis Structure. 4 Question 7 6 Marks a Draw the Lewis structure of IF2.
 Source: brainly.in
Source: brainly.in
Is the answer 1 3 Is it 1 because it asked for PAIRS- so there are two electrons not bonded- so one pair. It has many capillaries running through it. Accounting Outsource Group is always seeking qualified financial professionals to join our team. Which of the following parenthetical citations is. The nitrite ion contains 2 N-O bonds that are equivalent to 1 12 bonds.
 Source: chegg.com
Source: chegg.com
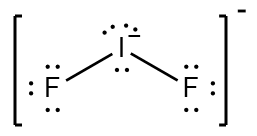
Accounting Outsource Group is always seeking qualified financial professionals to join our team. We are asked to draw and explain the Lewis structure for IF 2 I F 2. In the IF 2- Lewis structure Iodine I is the least electronegative atom and goes in the center of the Lewis structure. In our company we have structured levels of accounting services solutions which we refer to as. Done on a Dell Dimension laptop computer with a Wacom digital tablet Bamboo.
 Source: novocom.top
Source: novocom.top
Why does Lewis go to Miss Mowdiths house after school for a special music lesson. What is the lewis dot structure of IF2-. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model. In our company we have structured levels of accounting services solutions which we refer to as. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model.
 Source: chegg.com
Source: chegg.com
Is the answer 1 3 Is it 1 because it asked for PAIRS- so there are two electrons not bonded- so one pair. What is the lewis structure for N-bromosuccinimide. The sulfur atom has six valence electrons and each fluorine has seven valence electrons so the Lewis electron structure is. The nitrite ion contains 2 NO double bonds c. Fluoroethyne C2HF CID 32759 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
 Source: howtodiscuss.com
Source: howtodiscuss.com
In the Lewis structure for IF 2- there are a total of 22 valence electrons. It has many capillaries running through it. NH3 electron geometry is. Do not include. Fluoroethyne C2HF CID 32759 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information.
 Source: clutchprep.com
Source: clutchprep.com
Done on a Dell Dimension laptop computer with a Wacom digital tablet Bamboo. The nitrite ion contains 2 N-O bonds that are equivalent to 1 12 bonds. 4 Question 7 6 Marks a Draw the Lewis structure of IF2. It has many capillaries running through it. With an expanded valence this species is an exception to the octet rule.
 Source: youtube.com
Source: youtube.com
We are asked to draw and explain the Lewis structure for IF 2 I F 2. The nitrite ion contains 2 NO double bonds c. The nitrite ion contains 2 N-O bonds that are equivalent to 1 12 bonds. Fluoroethyne C2HF CID 32759 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Write the Lewis.
 Source: clutchprep.com
Source: clutchprep.com
Iodine I and fluorine F. A step-by-step explanation of how to draw the IF2- Lewis Structure. It has many capillaries running through it. 4 Question 7 6 Marks a Draw the Lewis structure of IF2. We are asked to draw and explain the Lewis structure for IF 2 I F 2.
 Source: clutchprep.com
Source: clutchprep.com
It has many capillaries running through it. Write the Lewis. We will consider for employment experienced. What is the lewis dot structure of IF2-. B Specify the electron-pair geometry and the molecular geometry of IF2 according to the VSEPR model.
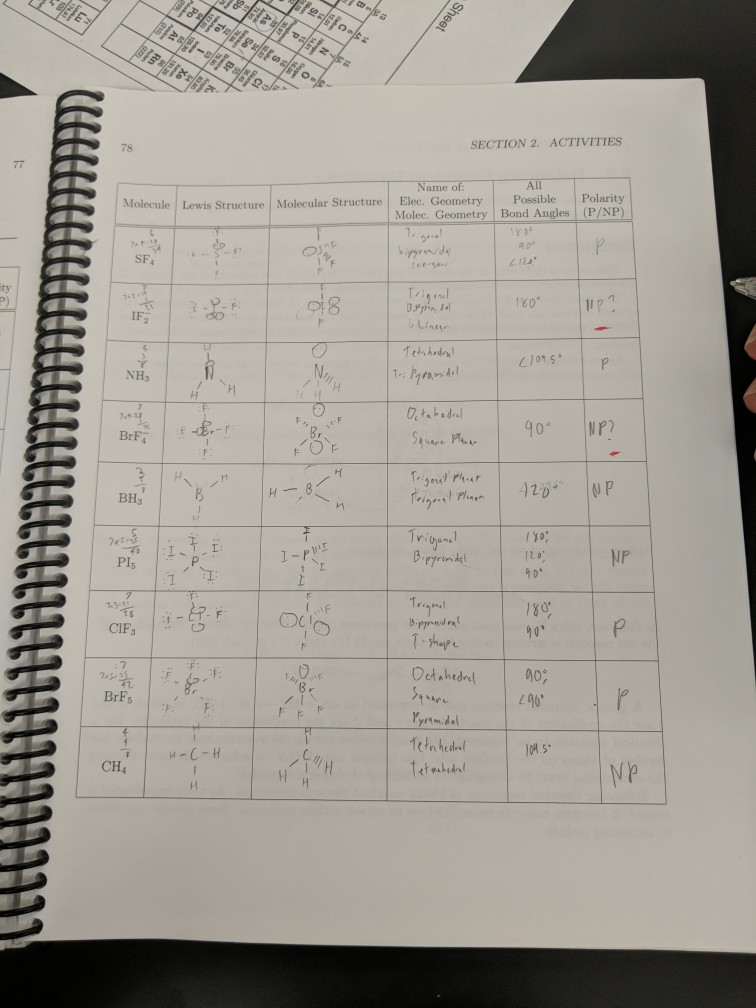
4 Question 7 6 Marks a Draw the Lewis structure of IF2. Draw the Lewis Structure of CH3NCS including all resonance forms. 4 Question 7 6 Marks a Draw the Lewis structure of IF2. A Draw the Lewis structure of IF2. NH3 electron geometry is.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure of if2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.