Lewis structure of so42
Lewis Structure Of So42. The Lewis Structure of any molecule helps to understand the bonding of atoms in the structure. Fri Sep 28 2018 729 am. 017 H 41 ö. Total of electrons 2 e - x 4 bonds 2 e - x 13 lone pairs.
 How To Draw The Lewis Structure Of So4 2 Sulfate Ion Youtube From youtube.com
How To Draw The Lewis Structure Of So4 2 Sulfate Ion Youtube From youtube.com
There are 32 valence electrons available for the Lewis structure for SO 42-. Mariam Baghdasaryan 4F Posts. There are no lone pairs in the last shell of sulfur atom. Let us begin by understanding the chemical bonding and molecular structure of sulfate. 12973487 15k 306k 703 Draw the Lewis dot structure of Hydrogen cyanide HCN molecule. Generally while forming bonds with other atoms.
It will hold more than 8 electrons.
70 More Lewis Dot Structures. 017 H 41 ö. O 30-4-03 Ola ö. Lewis Structures for N2. 12973480 141k 2820k 713 Draw the Lewis structure of iodine pentafluoride. It will hold more than 8 electrons.
 Source: novocom.top
Source: novocom.top
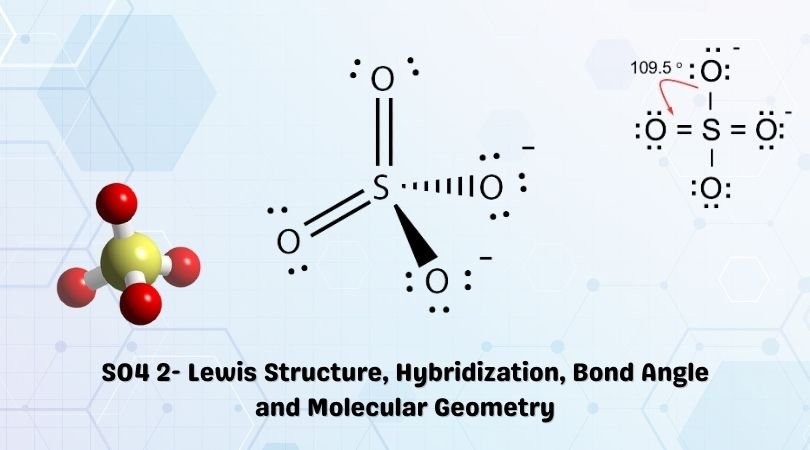
And these 2. All elements want an. SO4 2- Lewis Structure SO42- Lewis structure Lewis Structure for SO4 2- SO4 2- SO4 2- Electron Dot Structure Electron Dot Structure for SO4 2- How to D. For SO42- sulfate ion draw the Lewis structure by counting valence electrons of each atom. Remember Sulfur is in Period 3 and can hold more than 8 valence electrons.
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Formal Charge SO42-Moderators. For SO42- sulfate ion draw the Lewis structure by counting valence electrons of each atom. The Lewis structure for SO 42- is requires you to place more than 8 valence electrons on Sulfur S. O 5 O 4 O-1 O 2 Draw the Lewis structure for SO 2. 12973487 15k 306k 703 Draw the Lewis dot structure of Hydrogen cyanide HCN molecule.
 Source: chemistryscl.com
Source: chemistryscl.com
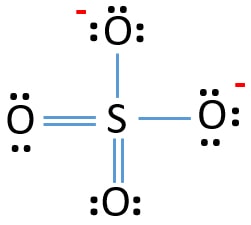
For example MgSO 4 is also known as Epsom Salts. This problem has been. 12973480 141k 2820k 713 Draw the Lewis structure of iodine pentafluoride. The reason is FORMAL CHARGE and. There are no lone pairs in the last shell of sulfur atom.
 Source: study.com
Source: study.com
Generally while forming bonds with other atoms. For SO42- sulfate ion draw the Lewis structure by counting valence electrons of each atom. The reason is FORMAL CHARGE and. All elements want an. Lets do the SO4 2- Lewis structure for the sulfate ion.
![]() Source: study.com
Source: study.com
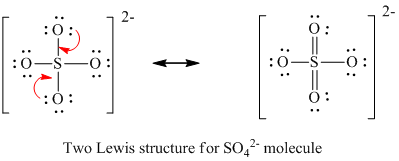
Therefore the total number of valence electrons in 6 46 2 32. Draw the Lewis structure for SO42. Total valence electrons concept is used to draw the lewis structure of SO42- ion. One is to follow the octet rule and having single bond for each oxygen each perfectly satisfies the octet rule. Find the Lewis Structure of the molecule.
 Source: chegg.com
Source: chegg.com
Fri Sep 28 2018 729 am. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo toothpaste etc. There are 32 valence electrons available for the Lewis structure for SO 42-. Formal Charge SO42-Post by Mariam Baghdasaryan 4F Mon Nov 05 2018 1207 am. Beside above why the Valency of so4 is 2.
 Source: youtube.com
Source: youtube.com
Fri Sep 28 2018 729 am. SO42 Lewis Structure As per the internal structure of a molecule we know that a molecule is composed of atoms which in turn is composed of a nucleus and electrons. This problem has been. Bent 3-Dimensional View of SO 2 Chemistry Home Dr. Formal Charge SO42-Post by Mariam Baghdasaryan 4F Mon Nov 05 2018 1207 am.
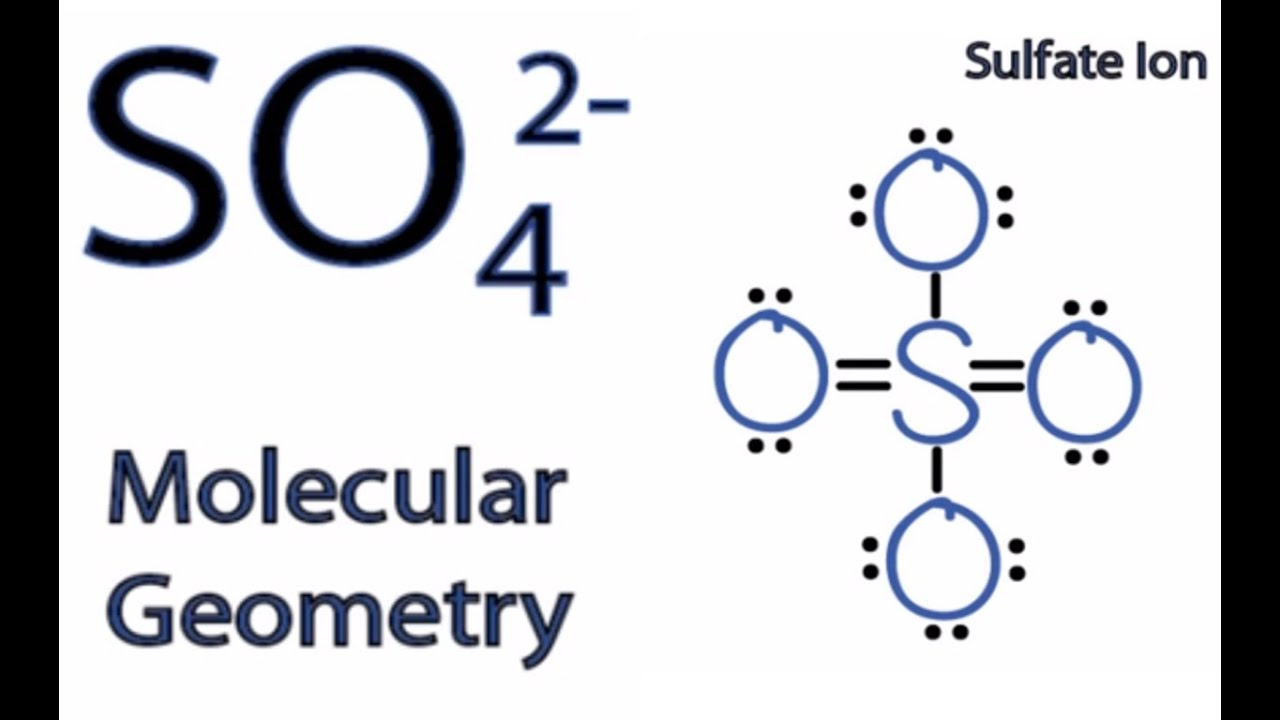 Source: youtube.com
Source: youtube.com
Bent 3-Dimensional View of SO 2 Chemistry Home Dr. A electron-domain geometry b molecular geometry c hybridization d Show the angle. For example MgSO 4 is also known as Epsom Salts. Lewis structure so42-. S does not follow the octet rule.
 Source: youtube.com
Source: youtube.com
017 H 41 ö. And these 2. Mariam Baghdasaryan 4F Posts. In the Lewis-dot structure the valance electrons are shown by dot. SO4 2- Lewis Structure SO42- Lewis structure Lewis Structure for SO4 2- SO4 2- SO4 2- Electron Dot Structure Electron Dot Structure for SO4 2- How to D.
 Source: youtube.com
Source: youtube.com
017 H 41 ö. Hence there are a total of 32 valence electrons for the Sulfate ion. Remember Sulfur is in Period 3 and can hold more than 8 valence electrons. It will hold more than 8 electrons. The given molecule is As we know that sulfur and oxygen has 6 valence electrons.
 Source: techiescientist.com
Source: techiescientist.com
Formal Charge SO42-Moderators. Formal Charge SO42-Moderators. O 5 O 4 O-1 O 2 Draw the Lewis structure for SO 2. Total of electrons 2 e - x 4 bonds 2 e - x 13 lone pairs. 70 More Lewis Dot Structures.
 Source: youtube.com
Source: youtube.com
Step-by-step tutorial for drawing the Lewis Structure for N2. 4 posts Page 1 of 1. Total valence electrons concept is used to draw the lewis structure of SO42- ion. Hence there are a total of 32 valence electrons for the Sulfate ion. Secondly what is the Lewis structure for so4 2.
 Source: techiescientist.com
Source: techiescientist.com
Find the Lewis Structure of the molecule. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo toothpaste etc. Fri Sep 28 2018 729 am. O 5 O 4 O-1 O 2 Draw the Lewis structure for SO 2. All elements want an.
 Source: study.com
Source: study.com
After determining how many valence electrons there are in NO2 place them around the central atom to complete the octets. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo toothpaste etc. The given molecule is As we know that sulfur and oxygen has 6 valence electrons. Total of electrons 2 e - x 4 bonds 2 e - x 13 lone pairs. 017 H 41 ö.
 Source: youtube.com
Source: youtube.com
Lewis structure so42-. The given molecule is As we know that sulfur and oxygen has 6 valence electrons. Remember Sulfur is in Period 3 and can hold more than 8 valence electrons. Therefore the total number of valence electrons in 6 46 2 32. You might think youve got the correct Lewis structure for SO 4 at first.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis structure of so42 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.