Magnesium bromide lewis structure
Magnesium Bromide Lewis Structure. Magnesium bromide MgBr 2 is a chemical compound of magnesium and bromine that is white and deliquescent. Magnesium bromide MgBr2 Br2Mg CID 522691 - structure chemical names physical and chemical properties classification patents literature biological. Mg 3t Br Which Lewis structure best obeys the octet rule for all of the atoms in the molecule and has formal charges that add to zero. It has to lose 2 electrons to attain a stable electronic configuration and octet valance shell structure resembling that of noble gases.
 Draw The Lewis Structure Of Mgbr2 Magnesium Bromide Youtube From youtube.com
Draw The Lewis Structure Of Mgbr2 Magnesium Bromide Youtube From youtube.com
It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. Mg 3t Br Which Lewis structure best obeys the octet rule for all of the atoms in the molecule and has formal charges that add to zero. Which is the correct Lewis structure for magnesium bromide. Magnesium bromide MgBr 2 is a chemical compound of magnesium and bromine that is white and deliquescent. Which one is not a valid Lewis symbol. All the four magnesium halides are known and are commercially available.
Benzylmagnesium Bromide is one of numerous organometallic magnesium compounds and Grignard reagents manufactured by American Elements under the trade name AE OrganometallicsOrganometallics are useful reagents catalysts and precursor materials with applications in thin film deposition industrial chemistry pharmaceuticals LED manufacturing and others.
The correct Lewis dot structure for magnesium bromide MgBr2 M g B r 2 is Br Mg Br with six dots. The correct Lewis dot structure for magnesium bromide M gBr2 M g B r 2 is Br - Mg - Br with six dots around each Br. All the four magnesium halides are known and are commercially available. Magnesium in its 2 oxidation state forms salts with a variety of counterions. Some substance identifiers may have been claimed. Which is the correct Lewis structure for magnesium bromide.
 Source: hd.codedwap.com
Source: hd.codedwap.com
W Ě -se- F Se. Today in this blog postI will show you magnesium bromide formula or formula of magnesium bromide in some steps. The correct Lewis dot structure for magnesium bromide MgBr2 M g B r 2 is Br Mg Br with six dots. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. Both of these salts have low solubility in organic solvents and a.
 Source: youtube.com
Source: youtube.com
- se- F Which is the worst Lewis structure for CNO based. Magnesium has an electronic configuration of 28. 2Which is the correct Lewis structure for magnesium bromide. Which is the correct Lewis structure for magnesium bromide. Magnesium bromide MgBr2 or Br2Mg CID 82241 - structure chemical names physical and chemical properties classification patents literature biological.
 Source: youtube.com
Source: youtube.com
Sorry for my horrible writing The structure should have single bond between. Magnesium in its 2 oxidation state forms salts with a variety of counterions. Today in this blog postI will show you magnesium bromide formula or formula of magnesium bromide in some steps. The Lewis Dot Structure for MgBr2 Magnesium Bromide is as follows. Benzylmagnesium Bromide is one of numerous organometallic magnesium compounds and Grignard reagents manufactured by American Elements under the trade name AE OrganometallicsOrganometallics are useful reagents catalysts and precursor materials with applications in thin film deposition industrial chemistry pharmaceuticals LED manufacturing and others.
 Source: novocom.top
Source: novocom.top
Bri 3rImg ed img3c. Benzylmagnesium Bromide is one of numerous organometallic magnesium compounds and Grignard reagents manufactured by American Elements under the trade name AE OrganometallicsOrganometallics are useful reagents catalysts and precursor materials with applications in thin film deposition industrial chemistry pharmaceuticals LED manufacturing and others. All the four magnesium halides are known and are commercially available. - se- F Which is the worst Lewis structure for CNO based. Magnesium in its 2 oxidation state forms salts with a variety of counterions.
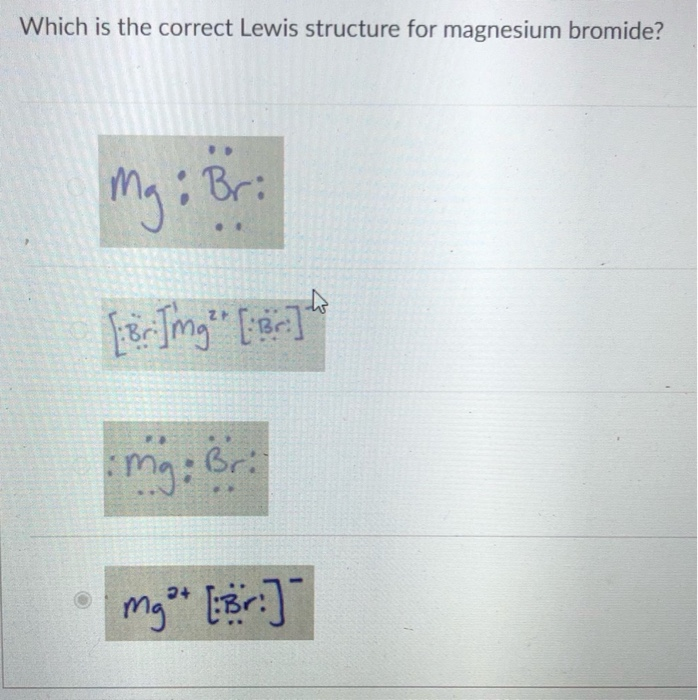 Source: chegg.com
Source: chegg.com
The Substance identity section is calculated from substance identification information from all ECHA databases. Magnesium has an electronic configuration of 28. Both of these salts have low solubility in organic solvents and a. The correct Lewis dot structure for magnesium bromide M gBr2 M g B r 2 is Br - Mg - Br with six dots around each Br. A lewis structure for the acetate ion is shown here Categories Chemistry chapter 9 and 10 Tags which is the correct lewis structure for magnesium bromide.
 Source: youtube.com
Source: youtube.com
Magnesium has an electronic configuration of 28. Magnesium in its 2 oxidation state forms salts with a variety of counterions. Of these magnesium bromide and iodide are often used as Lewis acids in a variety of stereoselective transformations. Magnesium has an electronic configuration of 28. The Substance identity section is calculated from substance identification information from all ECHA databases.
 Source: novocom.top
Source: novocom.top
Benzylmagnesium Bromide is one of numerous organometallic magnesium compounds and Grignard reagents manufactured by American Elements under the trade name AE OrganometallicsOrganometallics are useful reagents catalysts and precursor materials with applications in thin film deposition industrial chemistry pharmaceuticals LED manufacturing and others. 2Which is the correct Lewis structure for magnesium bromide. Of these magnesium bromide and iodide are often used as Lewis acids in a variety of stereoselective transformations. Transcribed image text. Magnesium bromide MgBr2 Br2Mg CID 522691 - structure chemical names physical and chemical properties classification patents literature biological.
 Source: chegg.com
Source: chegg.com
It has to lose 2 electrons to attain a stable electronic configuration and octet valance shell structure resembling that of noble gases. Magnesium has an electronic configuration of 28. All the four magnesium halides are known and are commercially available. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. Of these magnesium bromide and iodide are often used as Lewis acids in a variety of stereoselective transformations.
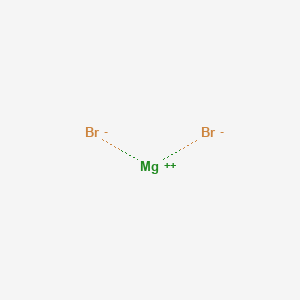
Today in this blog postI will show you magnesium bromide formula or formula of magnesium bromide in some steps. It is water-soluble and somewhat soluble in alcohol. W Ě -se- F Se. Magnesium in its 2 oxidation state forms salts with a variety of counterions. The substance identifiers displayed in the InfoCard are the best available substance name EC number CAS number andor the molecular and structural formulas.
 Source: novocom.top
Source: novocom.top
It is water-soluble and somewhat soluble in alcohol. Some substance identifiers may have been claimed. All the four magnesium halides are known and are commercially available. Which one is not a valid Lewis symbol. It is water-soluble and somewhat soluble in alcohol.
 Source: youtube.com
Source: youtube.com
The Substance identity section is calculated from substance identification information from all ECHA databases. - se- F Which is the worst Lewis structure for CNO based. All the four magnesium halides are known and are commercially available. Mg 3t Br Which Lewis structure best obeys the octet rule for all of the atoms in the molecule and has formal charges that add to zero. Which one is not a valid Lewis symbol.
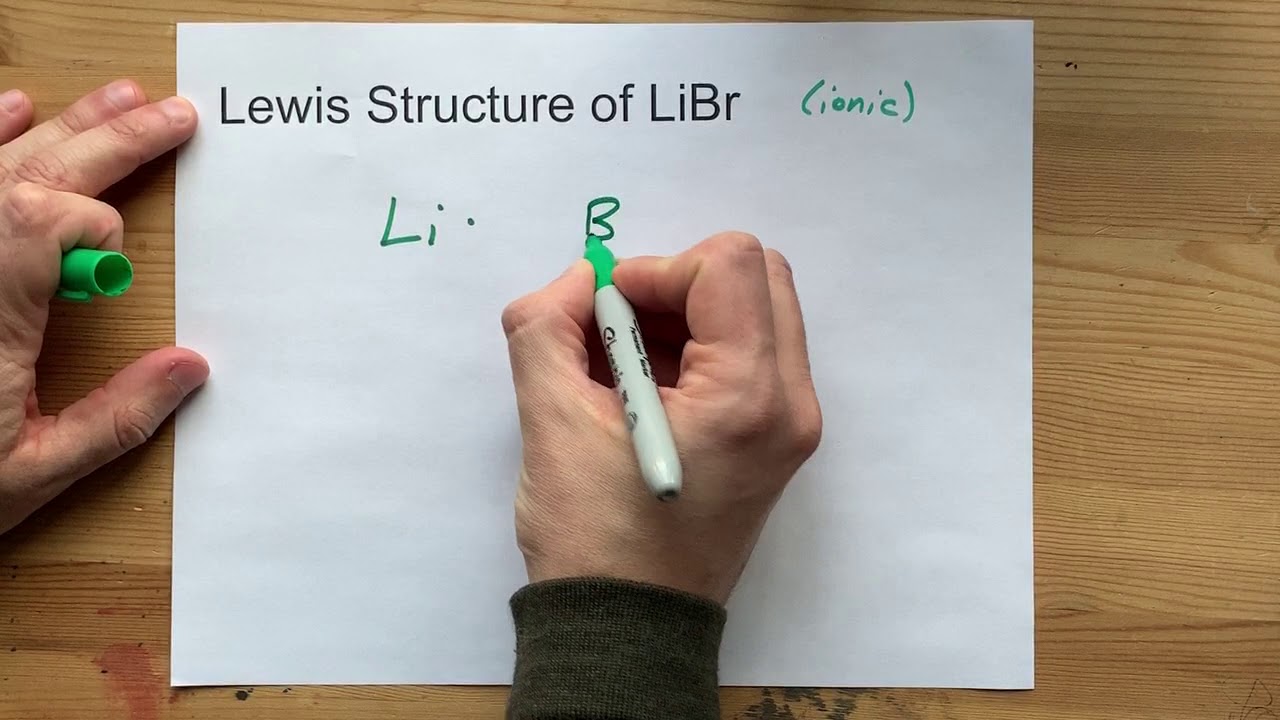 Source: youtube.com
Source: youtube.com
The Lewis Dot Structure for MgBr2 Magnesium Bromide is as follows. Which is the correct Lewis structure for magnesium bromide. Which is the correct Lewis structure for magnesium bromide. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It has to lose 2 electrons to attain a stable electronic configuration and octet valance shell structure resembling that of noble gases.
 Source: quora.com
Source: quora.com
A lewis structure for the acetate ion is shown here Categories Chemistry chapter 9 and 10 Tags which is the correct lewis structure for magnesium bromide. The substance identifiers displayed in the InfoCard are the best available substance name EC number CAS number andor the molecular and structural formulas. The correct Lewis dot structure for magnesium bromide MgBr2 M g B r 2 is Br Mg Br with six dots. Which one is not a valid Lewis symbol. Magnesium bromide MgBr 2 is a chemical compound of magnesium and bromine that is white and deliquescent.
 Source: clutchprep.com
Source: clutchprep.com
Magnesium from group 2A 2 valence electrons 2 charge lost 2 e- Bromine from group 7A 7 valence electrons -1 charge gained 1 e-85 210 ratings Problem Details. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. Magnesium from group 2A 2 valence electrons 2 charge lost 2 e- Bromine from group 7A 7 valence electrons -1 charge gained 1 e-85 210 ratings Problem Details. Which one is not a valid Lewis symbol. Transcribed image text.
 Source: youtube.com
Source: youtube.com
Transcribed image text. Magnesium bromide MgBr 2 is a chemical compound of magnesium and bromine that is white and deliquescent. Some substance identifiers may have been claimed. Which one is not a valid Lewis symbol. What is the correct Lewis dot diagram for MgBr2.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title magnesium bromide lewis structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






