Methyl acetate resonance structures
Methyl Acetate Resonance Structures. A major use of methyl acetate is as a volatile low toxicity solvent in glues paints and nail polish removers. 1 Do not exceed the octet on 2nd-row elements. Methyl acetate structure Structure of luciferin Ohtsuka et al 1976. It is classified as a hard base and is a base in the ECW model with E B 163 and C B 095.
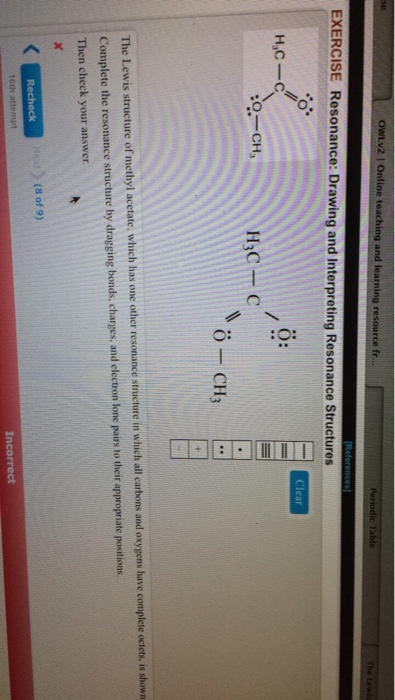
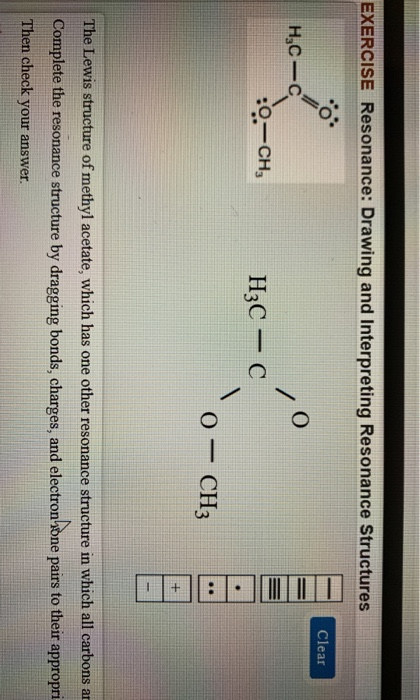
11 Nuclear magnetic resonance methyl acetate C Nuclear magnetic resonance spectrum acetaldehyde 732 acetophenone 732 anisole 672 benzaldehyde 732 benzoic acid 771 p-bromoacetophenone 449 2-butanone 449 732 crotonic acid. A major use of methyl acetate is as a volatile low toxicity solvent in glues paints and nail polish removers. Structure A structure B. H3C- H3C-C CH3 -CH3 А B Which structure contributes more to the resonance hybrid. The general formula of a carboxylic acid is RCOOH or RCO2H with R referring to the alkyl alkenyl aryl or other group. 1 Do not exceed the octet on 2nd-row elements.
Draw the linear azide anion N X 3 X.
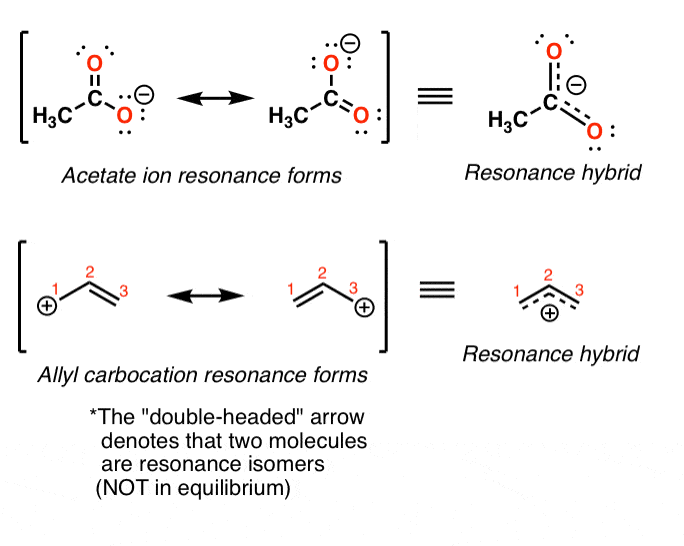
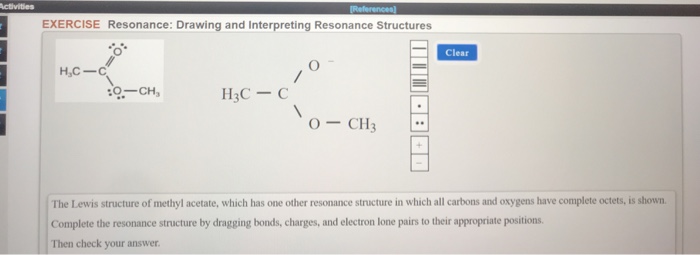
Methyl azide however looks completely different. When the structure of the same compound can be drawn with the different arrangements of electrons but the position of atoms remain same then the structures are. Lewis electron dot structure 1 is the major contributor to the hybrid since there are 8 electrons around each atom and there is no charge separation extra stability. Each of these arrows depicts the movement of two pi electrons. Methyl acetoacetate C5H8O3 CID 7757 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Draw three resonance contributors of methyl acetate an ester with the structure CH 3 COOCH 3 and order them according to their relative importance to the bonding picture of.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Deprotonation of a carboxylic acid gives a carboxylate anion. 2 Do not break single bonds. 771 cyclohexanol 634 cyclohexanone 732 ethyl benzoate 477 methyl acetate 443 methyl propanoate 450 methyl propyl ether 672. 11 Nuclear magnetic resonance methyl acetate C Nuclear magnetic resonance spectrum acetaldehyde 732 acetophenone 732 anisole 672 benzaldehyde 732 benzoic acid 771 p-bromoacetophenone 449 2-butanone 449 732 crotonic acid. It is classified as a hard base and is a base in the ECW model with E B 163 and C B 095.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The two proton groups in our methyl acetate sample are recorded as resonating at frequencies 205 and 367 ppm higher than TMS. Structure 2 is the least plausible contributor because there is charge separation and positive charge on an. 1 Do not exceed the octet on 2nd-row elements. Two must-follow rules when drawing resonance structures. 11 Nuclear magnetic resonance methyl acetate C Nuclear magnetic resonance spectrum acetaldehyde 732 acetophenone 732 anisole 672 benzaldehyde 732 benzoic acid 771 p-bromoacetophenone 449 2-butanone 449 732 crotonic acid.
 Source: chem.libretexts.org
Source: chem.libretexts.org
For an exercise on resonance structures its protonated form the guanidinium cation would be an interesting case and you might want to give it a try too. Golovnya RV Variation in retention indices and equivalent chain lengths of homologous series of n-alkyl acetates n-alkyl methyl ketones and methyl. Thus 205 ppm on this instrument corresponds to 615 Hz and 367 ppm corresponds to 1101 Hz. 771 cyclohexanol 634 cyclohexanone 732 ethyl benzoate 477 methyl acetate 443 methyl propanoate 450 methyl propyl ether 672. Rules for drawing resonance structures.
 Source: researchgate.net
Source: researchgate.net
Methyl acetate structure Structure of luciferin Ohtsuka et al 1976. The two resonance structures of methyl acetate are. As the name suggests there should be a methyl group C H X 3 and an azide group. One-millionth 10 ppm of 300 MHz is 300 Hz. When the structure of the same compound can be drawn with the different arrangements of electrons but the position of atoms remain same then the structures are.
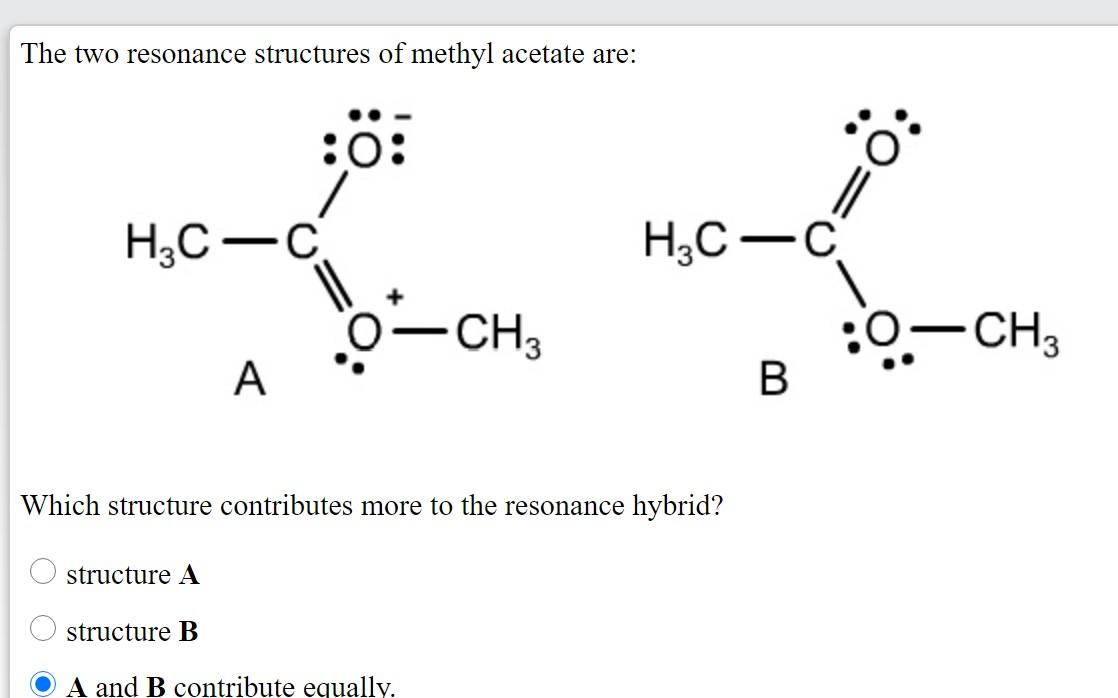
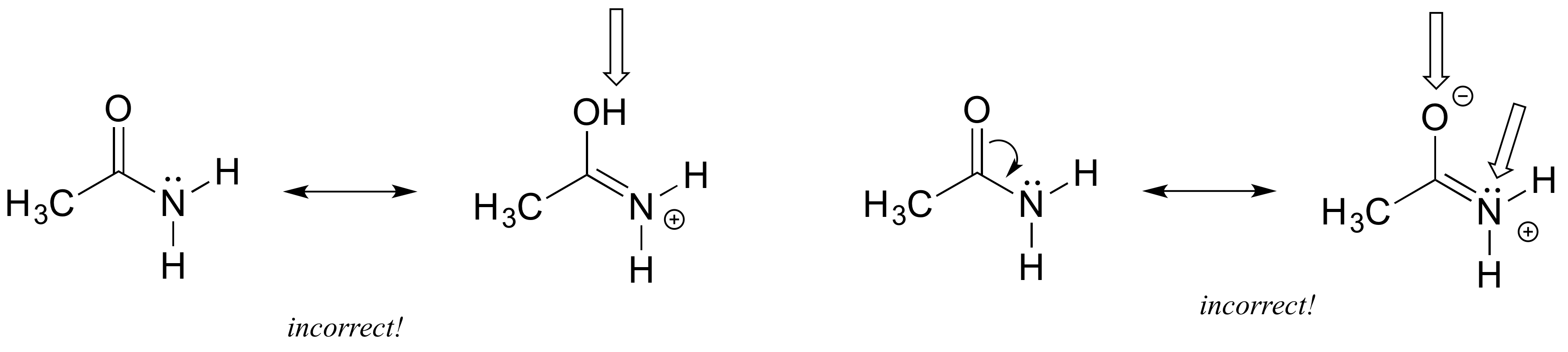
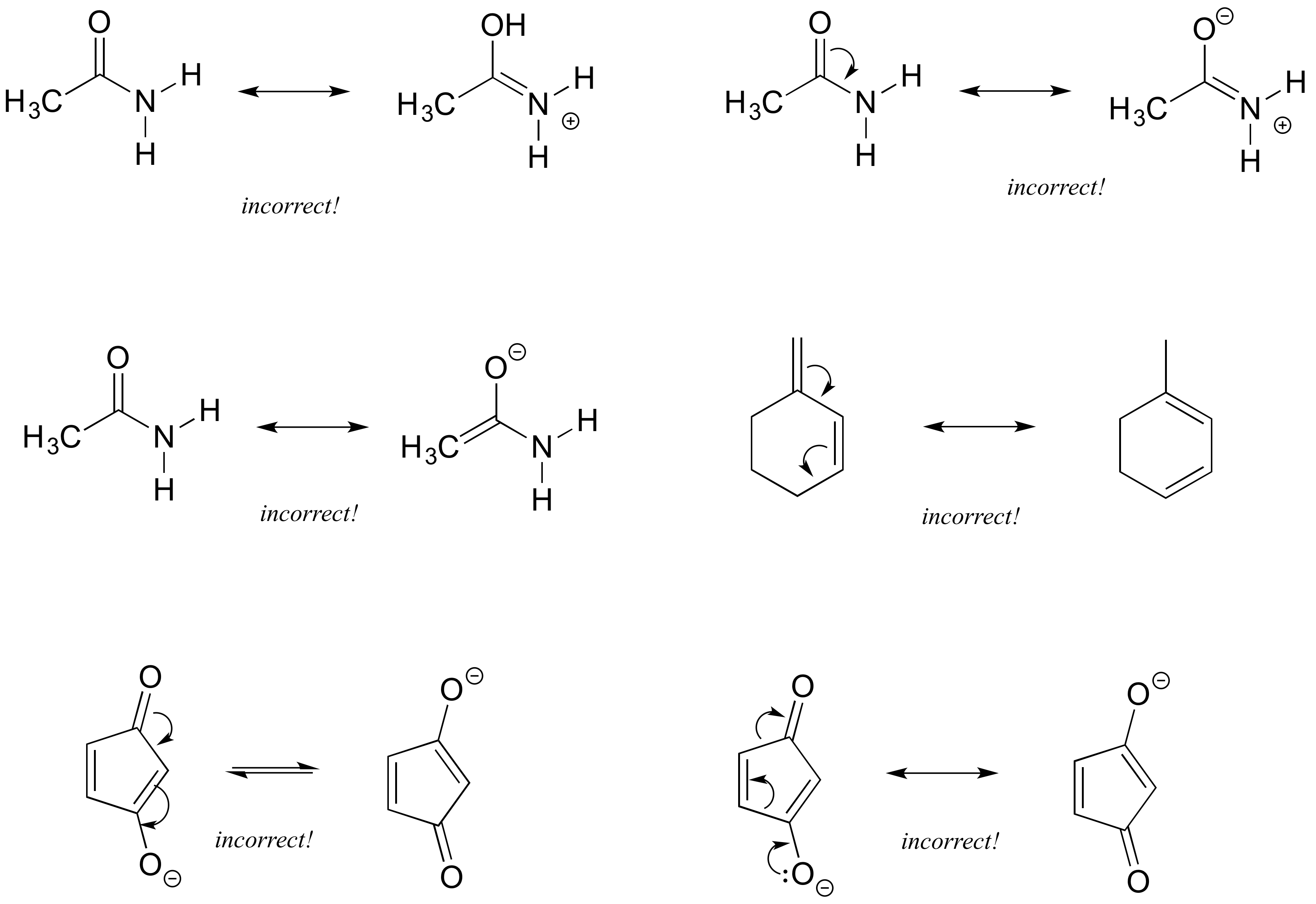
Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol. As the name suggests there should be a methyl group C H X 3 and an azide group. It is classified as a hard base and is a base in the ECW model with E B 163 and C B 095. Structures A and B are called resonance structures or resonance contributors. By convention resonance contributors are linked by a double-headed arrow and are sometimes enclosed by brackets.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
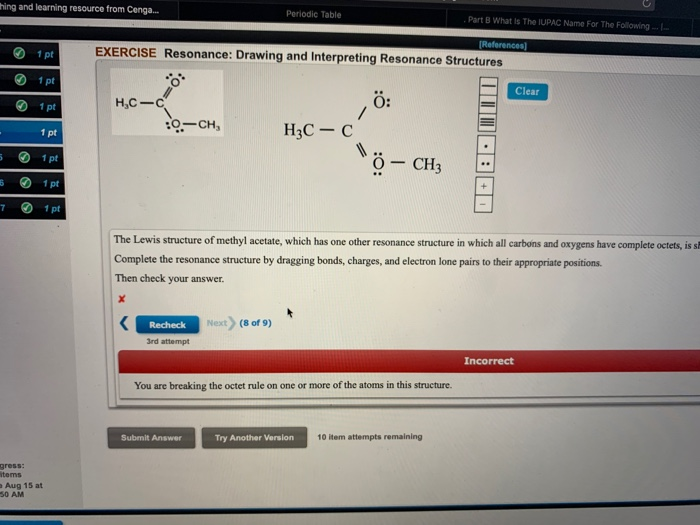
Draw three resonance contributors of methyl acetate an ester with the structure CH 3 COOCH 3 and order them according to their relative importance to the bonding picture of. Draw three resonance contributors of methyl acetate an ester with the structure CH 3 COOCH 3 and order them according to their relative importance to the bonding picture of. Structure 2 is the least plausible contributor because there is charge separation and positive charge on an. The second-row elements C N O F can only handle up to eight electrons because of their orbitals. Draw the Lewis structure including resonance structures for the acetate ion leftmathrmCH_3 mathrmCOO-right.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Draw three resonance contributors of methyl acetate an ester with the structure CH 3 COOCH 3 and order them according to their relative importance to the bonding picture of. The general formula of a carboxylic acid is RCOOH or RCO2H with R referring to the alkyl alkenyl aryl or other group. Structure 2 is the least plausible contributor because there is charge separation and positive charge on an. For an exercise on resonance structures its protonated form the guanidinium cation would be an interesting case and you might want to give it a try too. Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol.

The second-row elements C N O F can only handle up to eight electrons because of their orbitals. Thus 205 ppm on this instrument corresponds to 615 Hz and 367 ppm corresponds to 1101 Hz. 2 Do not break single bonds. Golovnya RV Variation in retention indices and equivalent chain lengths of homologous series of n-alkyl acetates n-alkyl methyl ketones and methyl. Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol.
 Source: chegg.com
Source: chegg.com
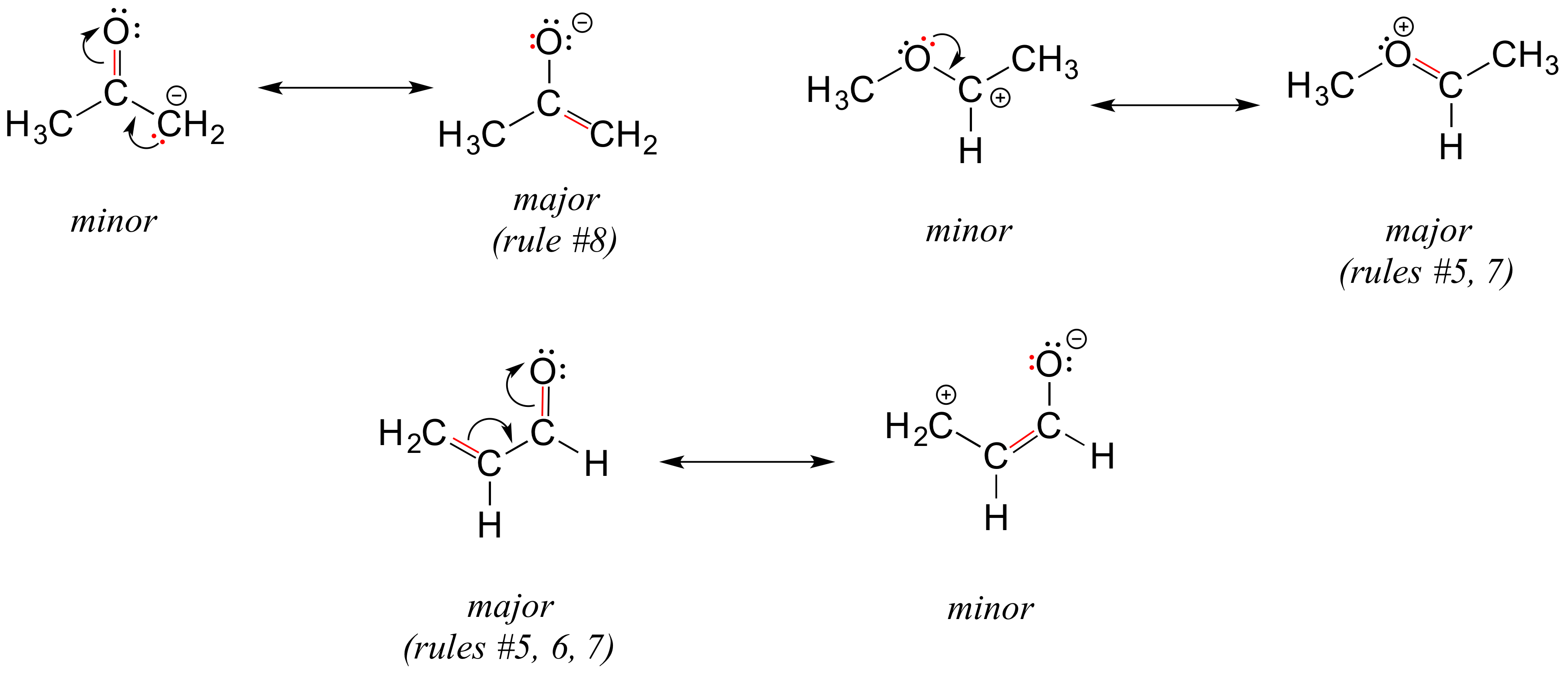
2 Do not break single bonds. 1 Do not exceed the octet on 2nd-row elements. In order to make it easier to visualize the difference between two resonance contributors small curved arrows are often used. Structure 2 is the least plausible contributor because there is charge separation and positive charge on an. A carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group.
 Source: youtube.com
Source: youtube.com
Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol. A carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. Draw the linear azide anion N X 3 X. A major use of methyl acetate is as a volatile low toxicity solvent in glues paints and nail polish removers. Methyl acetate is a Lewis base that forms 11 adducts with a variety of Lewis acids.
 Source: chem-net.blogspot.com
Source: chem-net.blogspot.com
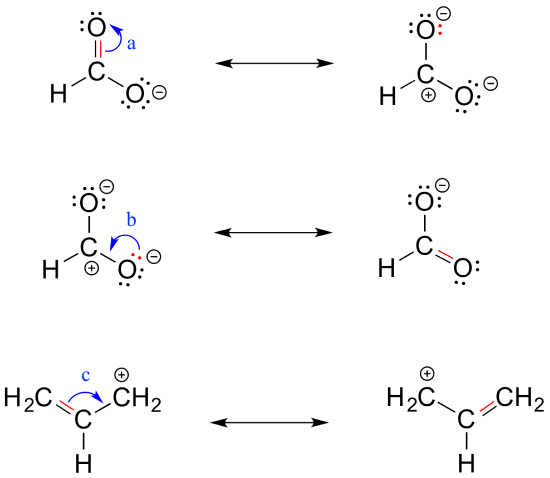
A low-boiling 57 colourless flammable liquid it is used as a solvent for many resins and oils. 2 Do not break single bonds. In order to make it easier to visualize the difference between two resonance contributors small curved arrows are often used. For each resonance structure ass Boost your resume with certification as an expert in up to 15 unique STEM subjects this summer. By convention resonance contributors are linked by a double-headed arrow and are sometimes enclosed by brackets.
 Source: chem-net.blogspot.com
Source: chem-net.blogspot.com
11 Nuclear magnetic resonance methyl acetate C Nuclear magnetic resonance spectrum acetaldehyde 732 acetophenone 732 anisole 672 benzaldehyde 732 benzoic acid 771 p-bromoacetophenone 449 2-butanone 449 732 crotonic acid. 2 Do not break single bonds. One-millionth 10 ppm of 300 MHz is 300 Hz. Lewis electron dot structure 1 is the major contributor to the hybrid since there are 8 electrons around each atom and there is no charge separation extra stability. Structure A structure B.
 Source: chegg.com
Source: chegg.com
Rules for drawing resonance structures. The general formula of a carboxylic acid is RCOOH or RCO2H with R referring to the alkyl alkenyl aryl or other group. When drawing different resonance structures remember. Rules for drawing resonance structures. Golovnya RV Variation in retention indices and equivalent chain lengths of homologous series of n-alkyl acetates n-alkyl methyl ketones and methyl.
 Source: chem.libretexts.org
Source: chem.libretexts.org
For an exercise on resonance structures its protonated form the guanidinium cation would be an interesting case and you might want to give it a try too. Structures A and B are called resonance structures or resonance contributors. Structure 2 is the least plausible contributor because there is charge separation and positive charge on an. H3C- H3C-C CH3 -CH3 А B Which structure contributes more to the resonance hybrid. The two proton groups in our methyl acetate sample are recorded as resonating at frequencies 205 and 367 ppm higher than TMS.
By convention resonance contributors are linked by a double-headed arrow and are sometimes enclosed by brackets. Show more umn class. The general formula of a carboxylic acid is RCOOH or RCO2H with R referring to the alkyl alkenyl aryl or other group. In order to make it easier to visualize the difference between two resonance contributors small curved arrows are often used. Important examples include the amino acids and fatty acids.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title methyl acetate resonance structures by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.