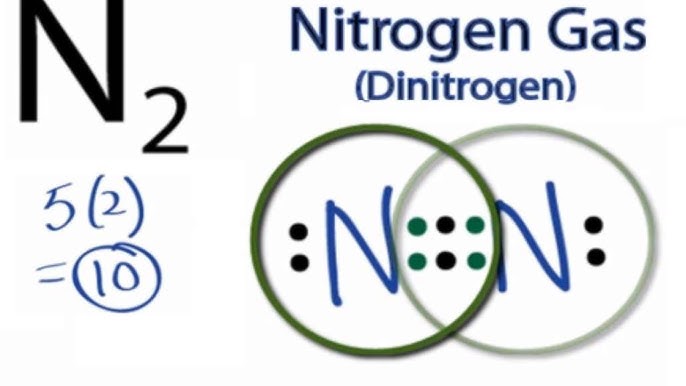
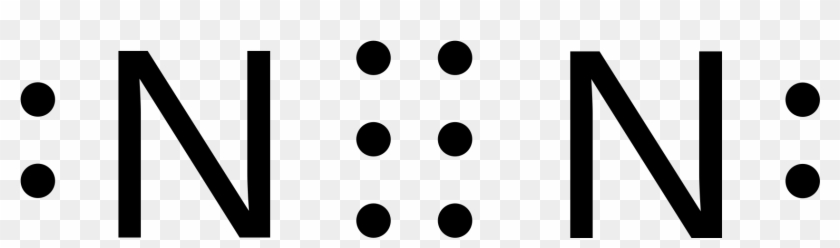
N2 lewis dot diagram
N2 Lewis Dot Diagram. First hopefully you noticed that magnesium nitride is an ionic compound since mg is an earth metal 2a column. A step-by-step explanation of how to draw the I2 Lewis Dot Structure Iodine GasFor the I2 structure use the periodic table to find the total number of val. Make sure you count the number of valence electrons correctly. Nitrogen is in group 5A also called Group 15.
 How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube From youtube.com
How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube From youtube.com
It also is a good example of a molecule with a triple bond. It shows the lone pairs of molecules existing in a molecule. We have two nitrogens. N2 Lewis structurenitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist.
The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible.
Since there are two Nitrogen atoms in N 2 you have a total of ten valence electrons to work with. I quickly take you through how to draw the lewis structure of n2 dinitrogen. Lewis dot diagram for n2. Lewis dot diagram for n2. 70 more lewis dot structures. We have two nitrogens.
 Source: techiescientist.com
Source: techiescientist.com
Each Nitrogen atom has five valence electrons. Each nitrogen atom also has a lone pair of electrons. I also go over the shape and bond angles. Lewis shows the diagram of the atomic bonding of the molecules or an element. A Lewis Structure can be drawn or presented both in case of the covalently bonded molecule and for the.
 Source: youtube.com
Source: youtube.com
Ntriple bondN and then two dots on each N in which ever spot is open. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist. Calculate the total valence electrons in no2 molecule. Lewis dot diagram for n2. It also is a good example of a molecule with a triple bond.
 Source: slidesharetips.blogspot.com
Source: slidesharetips.blogspot.com
The lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps. It also is a good example of a molecule with a triple bond. N2 Lewis structurenitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. If you are talking about the lewis dot diagram then n2 would have 5 dots around each of the letter ns so that there would be 6 dots total triple bond between the two ns and a pair of dots. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom.
 Source: quora.com
Source: quora.com
The lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps. Considering the energy level diagram the configuration of N2 is σ1S2 σ 1S2 σ2S2 σ2S2 π2Px2 π2Py2 σ2Pz1. The Lewis Structure or the Lewis Dot Structure or the Lewis Dot Diagram named after Gilbert N. Since N is a member of the Group 5A based on the periodic table the number of electrons in its outermost shell must be 5. Lewis electron dot diagrams for ions have fewer for cations or more for anions dots than the corresponding atom.
 Source: youtube.com
Source: youtube.com
To draw the N2 Lewis structure we have to find out the valence electrons of nitrogen firstWe express valence electrons as dots in lewis dot structure. Considering the energy level diagram the configuration of N2 is σ1S2 σ 1S2 σ2S2 σ2S2 π2Px2 π2Py2 σ2Pz1. Calculate the total valence electrons in no2 molecule. It also is a good example of a molecule with a triple bond. Each Nitrogen atom has five valence electrons.
 Source: socratic.org
Source: socratic.org
Lewis dot diagram for n2. N2 Lewis structurenitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. Since N is a member of the Group 5A based on the periodic table the number of electrons in its outermost shell must be 5. Considering the energy level diagram the configuration of N2 is σ1S2 σ 1S2 σ2S2 σ2S2 π2Px2 π2Py2 σ2Pz1. Nitrogen is in group 5A also called Group 15.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
Conclusion In the Lewis structure of the N2 molecule there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen. N2 Lewis Structure Valence ElectronsPolar or Non polar. To draw the N2 Lewis structure we have to find out the valence electrons of nitrogen firstWe express valence electrons as dots in lewis dot structure. Multiply those together we have a total of 10 valence electrons for the n2 lewis structure. Lewis dot diagram for n2.
 Source: study.com
Source: study.com
If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter Ns so that there would be 6 dots total What is the Lewis dot structure for N2 look like. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible. Calculate the total valence electrons in no2 molecule. N2 Lewis structurenitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots.
 Source: socratic.org
Source: socratic.org
Each Nitrogen atom has five valence electrons. The lewis dot structure for any molecule can be found by following a general set of rules consisting of 5 or sometimes 6 steps. 11 N2 Lewis Structure. The remaining lone pairs are repulsed by this dense electronegativity and so are drawn to appear as far away as possible. First hopefully you noticed that magnesium nitride is an ionic compound since mg is an earth metal 2a column.
 Source: quora.com
Source: quora.com
We have two nitrogens. Make sure you count the number of valence electrons correctly. Hellotoday I am going to draw the N2 Lewis structure in just few steps. It shows the lone pairs of molecules existing in a molecule. A Lewis Structure can be drawn or presented both in case of the covalently bonded molecule and for the.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
A step-by-step explanation of how to draw the Ba3N2 Lewis Dot StructureFor Ba3N2 we have an ionic compound and we need to take that into account when we dra. I also go over the shape and bond angles. Each nitrogen atom also has a lone pair of electrons. Each Nitrogen atom has five valence electrons. Here is the electron dot structure for a single N atom.
 Source: youtube.com
Source: youtube.com
Here is the electron dot structure for a single N atom. Considering the energy level diagram the configuration of N2 is σ1S2 σ 1S2 σ2S2 σ2S2 π2Px2 π2Py2 σ2Pz1. Drawing the Lewis Structure for N 2. Nitrogen is in group 5A also called Group 15. If you are talking about the lewis dot diagram then n2 would have 5 dots around each of the letter ns so that there would be 6 dots total triple bond between the two ns and a pair of dots.
 Source: clipartmax.com
Source: clipartmax.com
Lewis dot diagram for n2. Ntriple bondN and then two dots on each N in which ever spot is open. Ionic compounds are formed by the metals giving electrons to the nonmetals. N2 Lewis Structure Valence ElectronsPolar or Non polar. Lewis shows the diagram of the atomic bonding of the molecules or an element.
 Source: hndassignmenthelp.com
Source: hndassignmenthelp.com
If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter Ns so that there would be 6 dots total What is the Lewis dot structure for N2 look like. If you are talking about the Lewis Dot Diagram then N 2 would have 5 dots around each of the letter Ns so that there would be 6 dots total What is the Lewis dot structure for N2 look like. A step-by-step explanation of how to draw the I2 Lewis Dot Structure Iodine GasFor the I2 structure use the periodic table to find the total number of val. Make sure you count the number of valence electrons correctly. Lewis dot diagram for n2.
 Source: youtube.com
Source: youtube.com
Ntriple bondN and then two dots on each N in which ever spot is open. Each Nitrogen atom has five valence electrons. Nitrogen Fixing Plants to Fertilize Tea Organically Alex. I quickly take you through how to draw the lewis structure of n2 dinitrogen. Lewis shows the diagram of the atomic bonding of the molecules or an element.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title n2 lewis dot diagram by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.