Orbital notation for lithium
Orbital Notation For Lithium. Lithium was used during the 19th century to treat gout. Four electrons fill both the 1s and 2s orbitals. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. ALithium - Z 3.
 Dublin Schools Lesson Orbital Diagrams And Electron Configurations From dashboard.dublinschools.net
Dublin Schools Lesson Orbital Diagrams And Electron Configurations From dashboard.dublinschools.net
Orbital Filling Diagrams and Electron Configurations. Lithium salts such as lithium carbonate Li2CO3 lithium citrate and lithium. Orbital notation is a way of writing an electron configuration to provide more specific information about the electrons in an atom of an element. Orbital notation. Four electrons fill both the 1s and 2s orbitals. When we write the configuration well put all 20 electrons in orbitals around the nucleus of.
The electron configuration of lithium is 1 s2 2 s1.
For lithium the outer most electron is in the 2s orbital for sodium the 3s for potassium the 4s etc. According to the Aufbau process sublevels and orbitals are filled with electrons in order of increasing energy. The net charge of lithium atom is zero the charge of the unpaired 2s electron is obviously e. The electron configuration of lithium is 1 s2 2 s1. This electron is located on the second energy level in the 2s-orbital. Electrons are fermions so the unpaired 2 s electron has the spin 12.

Now that we have filled the 1s shell we move to the 2nd energy level and start to work on the second shell with lithium. Orbital Notation - Pictures Using the periodic table from the previous slide we can also create picture representations of the electron configuration called orbital notation We use arrows to represent the electrons Remember those three rules. Now that we have filled the 1s shell we move to the 2nd energy level and start to work on the second shell with lithium. Lithium Cation is a monovalent cation that is metabolized much like sodium and is important in many cellular functions inside or on the surface of cells. Occupants of orbitals have parallel same direction spins and are assigned an up arrow.
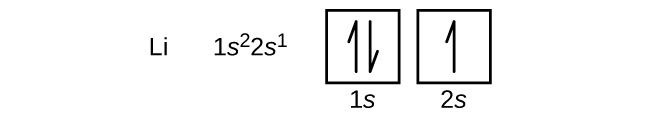 Source: chem.libretexts.org
Source: chem.libretexts.org
You can see that the oxygen atom has eight electrons 6 of its own and one from each lithium and the two lithium atoms have two electrons each. As shown previously the 2 s orbital is lower in energy than the 2 p orbitals so the electron configuration of lithium in spdf notation and orbital box notation is. Orbital Filling Diagrams and Electron Configurations. Lithium was used during the 19th century to treat gout. Since the s sublevel consists of just one orbital the second electron simply pairs up with the first electron as in helium.
 Source: dashboard.dublinschools.net
Source: dashboard.dublinschools.net
The electron configuration of lithium is 1s²2s¹. 1s 2 2s 1. The second electron to enter the orbital thus forming an electron pair is assigned a down arrow to represent opposite spin. Oxygen likes to have two additional electrons to make it happy. Orbital notation.
 Source: intl.siyavula.com
Source: intl.siyavula.com
Helium has two electrons. According to the Aufbau process sublevels and orbitals are filled with electrons in order of increasing energy. Orbital notation. We say the general electron configuration for the alkali metals is ns 1. 1s 2 2s 2 2p 1.
 Source: youtube.com
Source: youtube.com
The orbital filling diagram of lithium. Now that we have filled the 1s shell we move to the 2nd energy level and start to work on the second shell with lithium. The electron configuration of lithium is 1 s 2 2 s 1. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Orbital notation.
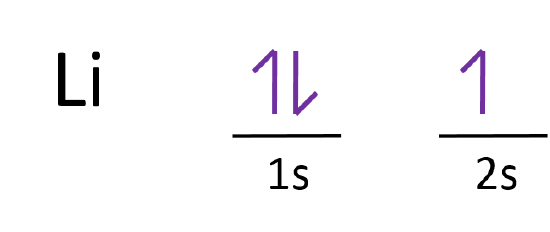 Source: chem.libretexts.org
Source: chem.libretexts.org
Boron atomic number 5 has five electrons. Smyk who gave me this ppt. Here is a video which explains how to write orbital notation for the element krypton. Orbital notation. The electron configuration of lithium is 1 s 2 2 s 1.

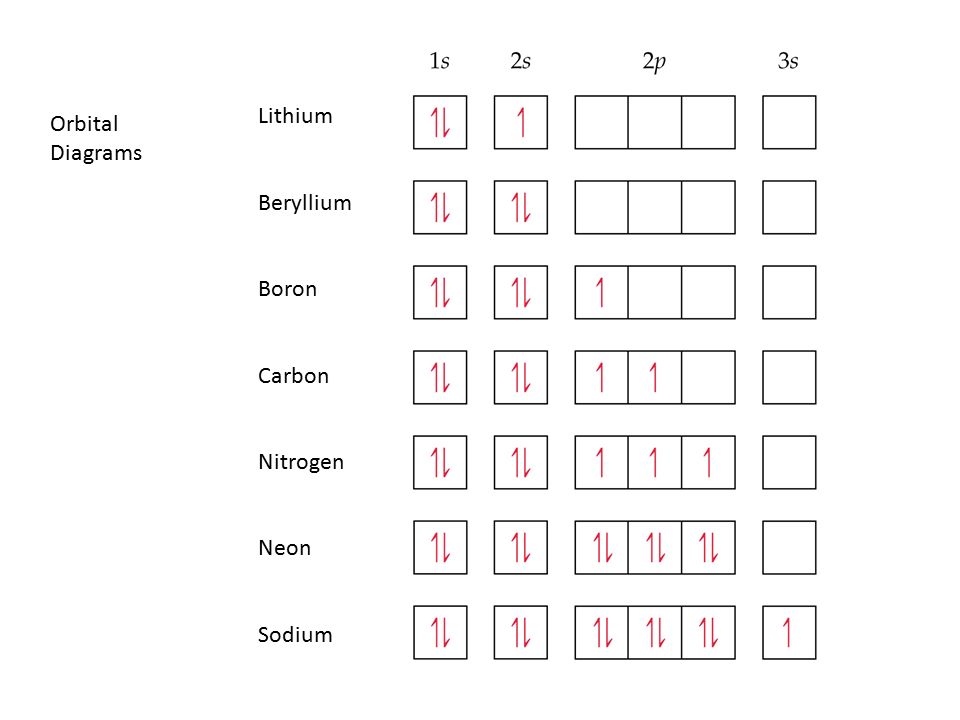
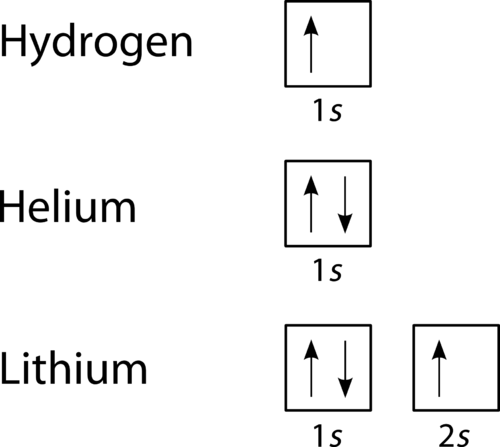
This means that there are two electrons in the 1s orbital and one electron in the higher energy 2s orbital. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Four electrons fill both the 1s and 2s orbitals. The electron configuration of lithium is 1 s 2 2 s 1. Orbital filling diagrams for hydrogen helium and lithium.
 Source: slideplayer.com
Source: slideplayer.com
Four electrons fill both the 1s and 2s orbitals. Oxygen likes to have two additional electrons to make it happy. What is Hunds Rule. The electron configuration of lithium is 1s²2s¹. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital.
 Source: sansona.github.io
Source: sansona.github.io
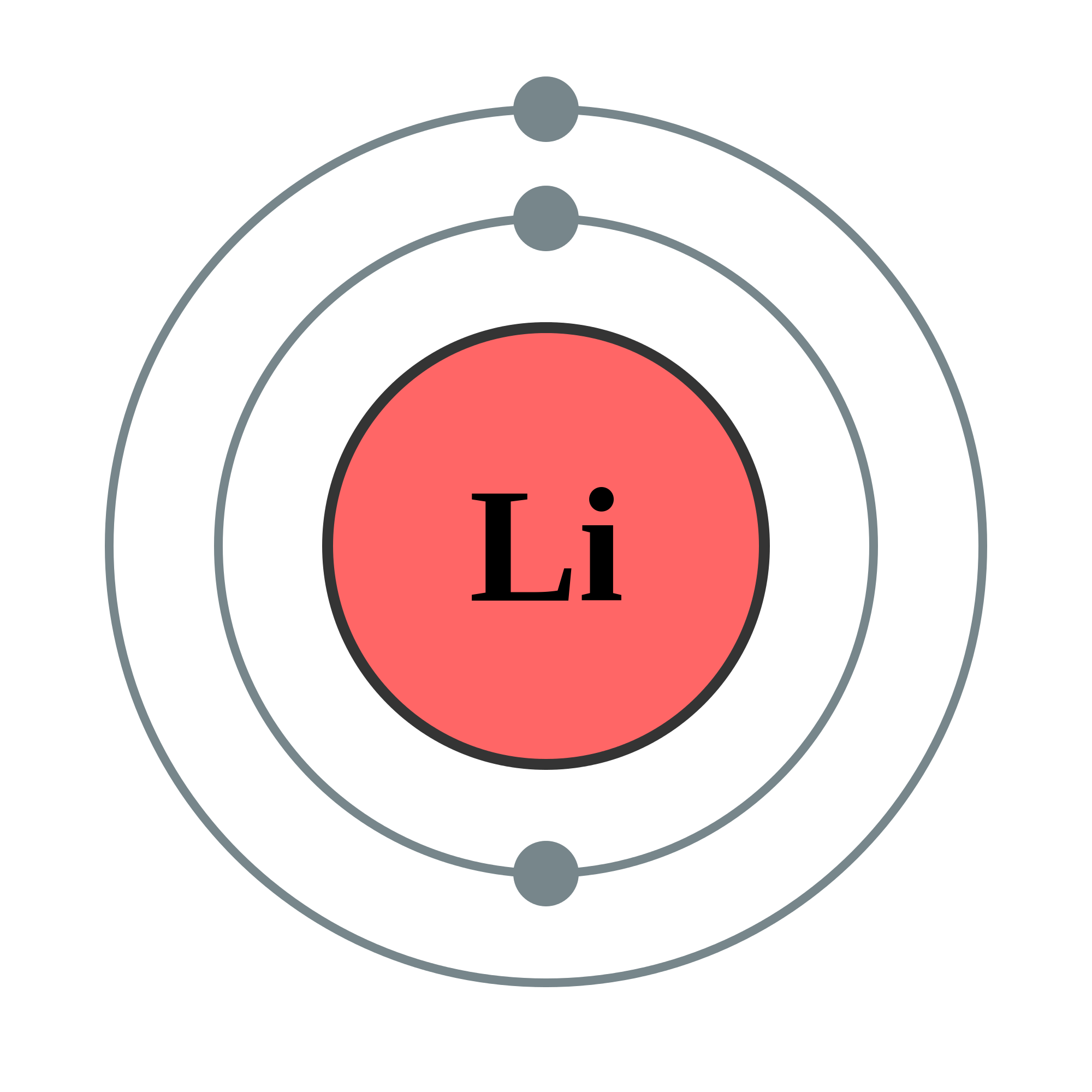
Lithium and Beryllium - The 2s Orbital. Lithium to Neon Lithium has three electrons two in the 1s orbital and one that is in an orbital in the second energy level. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. When we write the configuration well put all 20 electrons in orbitals around the nucleus of. Oxygen likes to have two additional electrons to make it happy.
 Source: youtube.com
Source: youtube.com
All of the alkali metals have the outer most or valence electron in an s orbital. Lithium to Neon Lithium has three electrons two in the 1s orbital and one that is in an orbital in the second energy level. The net charge of lithium atom is zero the charge of the unpaired 2s electron is obviously e. Lithium salts such as lithium carbonate Li2CO3 lithium citrate and lithium. According to the Aufbau process sublevels and orbitals are filled with electrons in order of increasing energy.
 Source: chem.fsu.edu
Source: chem.fsu.edu
You can see that the oxygen atom has eight electrons 6 of its own and one from each lithium and the two lithium atoms have two electrons each. Lithium to Neon Lithium has three electrons two in the 1s orbital and one that is in an orbital in the second energy level. Now that we have filled the 1s shell we move to the 2nd energy level and start to work on the second shell with lithium. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. The periodic table displayed uses color to denote the location of the outer-most electrons.
 Source: wps.prenhall.com
Source: wps.prenhall.com
The periodic table displayed uses color to denote the location of the outer-most electrons. Lithium was used during the 19th century to treat gout. Now the lithium cation Li is formed when lithium loses the electron located on its outermost shell its valence electron. Lithium has a configuration of 1s 2 2s 1. Boron atomic number 5 has five electrons.

According to the Aufbau process sublevels and orbitals are filled with electrons in order of increasing energy. The electron configuration of lithium is 1 s2 2 s1. What is Hunds Rule. The spin of a lithium nucleus depends on the isotope. Smyk who gave me this ppt.
 Source: study.com
Source: study.com
Now that we have filled the 1s shell we move to the 2nd energy level and start to work on the second shell with lithium. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The lowest energy orbital 1s has enough room to accommodate both electrons so Helium has a 1s 2 configuration. Boron atomic number 5 has five electrons.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Orbital notation. Occupants of orbitals have parallel same direction spins and are assigned an up arrow. Therefore the Li electron configuration will be 1s 2 2s 1. The sum of the superscripts in an electron configuration is equal to the number of electrons in that atom which is in turn equal to its atomic number. PURPOSE In this activity you will acquire an ability to write electron configurations orbital notations and a set of.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title orbital notation for lithium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





