Phosphorus pentafluoride lewis structure
Phosphorus Pentafluoride Lewis Structure. In this molecule of Phosphorus Pentachloride the five valance electrons surrounding phosphorus are being bonded with 5 atoms of Chloride which all have 7 valance electrons. Phosphorus pentafluoride PF 5 is a phosphorus halide. Phosphorus pentafluoride P F 5 is a phosphorus halide. The electronegativity of fluorine is greater than that of phosphorusso the phosphorus atom is placed in the center of the molecule.
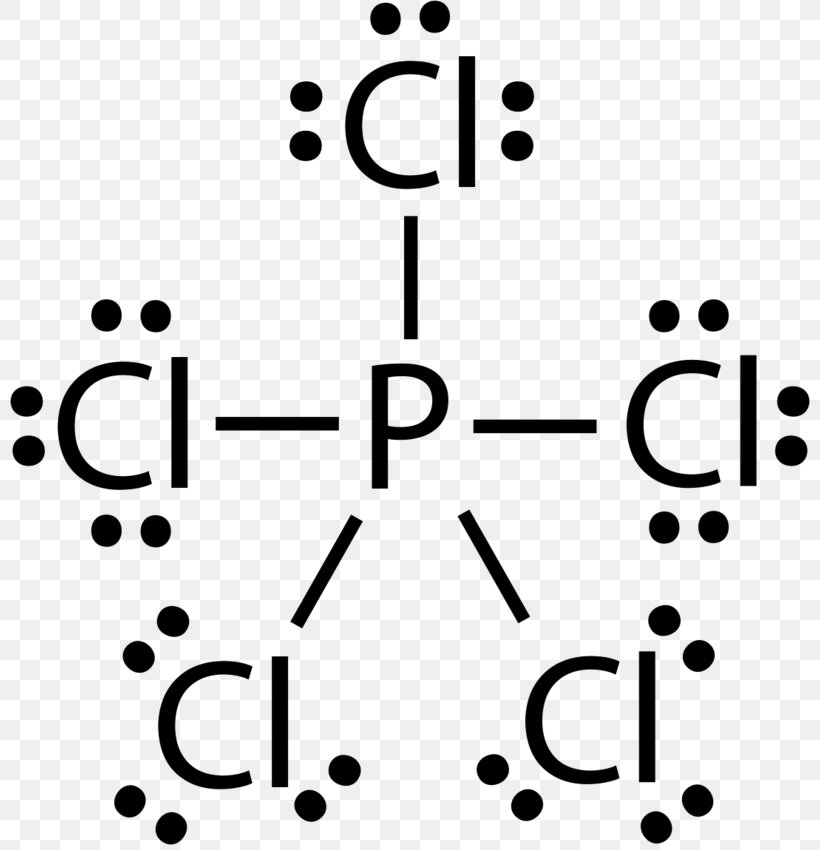 Lewis Structure Phosphorus Trichloride Phosphorus Pentachloride Phosphorus Pentafluoride Png 800x850px Lewis Structure Area Atom Black And From favpng.com
Lewis Structure Phosphorus Trichloride Phosphorus Pentachloride Phosphorus Pentafluoride Png 800x850px Lewis Structure Area Atom Black And From favpng.com
Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride which remains a favored method. Phosphorus pentafluoride P F 5 is a phosphorus halide. It is a colourless toxic gas that fumes in air. To draw the PF5 Lewis structure follow the below instructions. The fluorine is in group 7 and has seven valence electrons. It is toxic to inhale.
Pf5 lewis structure phosphorus pentafluoride draw structures octet rule phosphorous obey chemistry expanded valence chem sulfur.
The Phosphorus Pentafluoride PF5. The electronegativity of fluorine is greater than that of phosphorusso the phosphorus atom is placed in the center of the molecule. Phosphorus pentafluoride PF 5 is a phosphorus halide. Toxic and corrosive fumes are. Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride which remains a favored method. The reaction of the 45-dichloro-13-dimesitylimidazol-2-ylidene 148 with arsenic pentafluoride in 13-bistrifluoromethylbenzene furnished the 13-dimesityl-45-dichloroimidazolium-2-pentafluoroarsenate 150 in 65 yield Scheme 34.
 Source: youtube.com
Source: youtube.com
Phosphorus Pentafluoride on Wikipedia. Molecular Geometry Polarity Tutorial. Phosphorus Pentafluordie is a colourless and toxic gas. Phosphorus pentafluoride is a colorless poisonous nonflammable compressed gas with a pungent odor. Single-crystal X-ray studies indicate PF5 molecule has two distinct PF bonds axial and equatorial.
 Source: scienceofstudying.blogspot.com
Source: scienceofstudying.blogspot.com
Lewis structures phosphorus pentafluoride pf5 molecular pf vsepr dimensional three formula sundin. All the five fluorine atoms lie symmetric to the central phosphorus atom. But from PF5 formula it means that five fluorine so multiple 75 which. Phosphorus pentafluoride PF 5 is a phosphorus halide. Therefore this molecule is nonpolar.
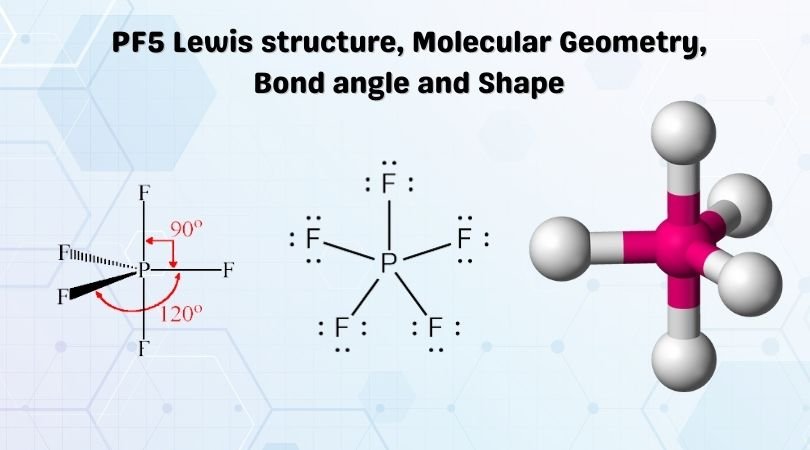 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Single-crystal X-ray studies indicate PF5 molecule has two distinct PF bonds axial and equatorial. This molecule is also known as the halide gas as it consists of Fluorine a halogen atom. First of all find out the total number of valence electrons in the PF5 using the periodic table. Phosphorus pentafluoride PF 5 is a phosphorus halide. Toxic and corrosive fumes are.
 Source: youtube.com
Source: youtube.com
Molecular Geometry Polarity Tutorial. Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride which remains a favored method. Phosphorus pentafluoride P F 5 is a phosphorus halide. Lewis Structure of Phosphorus Pentachloride. The fluorine is in group 7 and has seven valence electrons.
 Source: people.uwplatt.edu
Source: people.uwplatt.edu
PF5 Lewis StructureLewis Structure of PF5 Phosphorus PentafluorideDraw Lewis Structure for PF5. Pf5 lewis structure phosphorus pentafluoride draw structures octet rule phosphorous obey chemistry expanded valence chem sulfur. Phosphorus Pentafluoride is used as a fluorinating agent used in various industrial chemical reactions. Draw the Lewis structure for phosphorus pentafluoride PF 5. Phosphorus pentafluoride P F 5 is a phosphorus halide.
 Source: favpng.com
Source: favpng.com
Phosphorus pentafluoride Wikidata Phosphorus pentafluoride Wikipedia PPT Molecular Compounds Chapter 8 PowerPoint Lewis Structure What is the hybridization of the central phosphorus atom 188 Occurrence Preparation and Properties of Phosphorus Lewis Structure For Pf5 видео WikiBitme Exceptions to the Octet Rule ChemPaths The. A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot. PFax 1580 pm and PFeq 1522 pm. The compound is nonpolar in nature because of the symmetric geometrical structure. Phosphorus pentafluoride PF 5 is a phosphorus halide.
 Source: pngegg.com
Source: pngegg.com
It is a colourless toxic gas that fumes in air. Phosphorus Pentafluoride on Wikipedia. But from PF5 formula it means that five fluorine so multiple 75 which. Phosphorus pentafluoride Wikidata Phosphorus pentafluoride Wikipedia PPT Molecular Compounds Chapter 8 PowerPoint Lewis Structure What is the hybridization of the central phosphorus atom 188 Occurrence Preparation and Properties of Phosphorus Lewis Structure For Pf5 видео WikiBitme Exceptions to the Octet Rule ChemPaths The. It is a colourless toxic gas that fumes in air.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is a colourless toxic gas that fumes in air. It is toxic to inhale. Phosphorus pentafluoride Wikidata Phosphorus pentafluoride Wikipedia PPT Molecular Compounds Chapter 8 PowerPoint Lewis Structure What is the hybridization of the central phosphorus atom 188 Occurrence Preparation and Properties of Phosphorus Lewis Structure For Pf5 видео WikiBitme Exceptions to the Octet Rule ChemPaths The. This molecule is also known as the halide gas as it consists of Fluorine a halogen atom. In this molecule of Phosphorus Pentachloride the five valance electrons surrounding phosphorus are being bonded with 5 atoms of Chloride which all have 7 valance electrons.
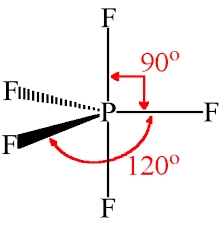 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Pf5 lewis structure phosphorus pentafluoride draw structures octet rule phosphorous obey chemistry expanded valence chem sulfur. Lewis Structure of Phosphorus Pentachloride. PFax 1580 pm and PFeq 1522 pm. Phosphorus pentafluoride Wikidata Phosphorus pentafluoride Wikipedia PPT Molecular Compounds Chapter 8 PowerPoint Lewis Structure What is the hybridization of the central phosphorus atom 188 Occurrence Preparation and Properties of Phosphorus Lewis Structure For Pf5 видео WikiBitme Exceptions to the Octet Rule ChemPaths The. It is very toxic by inhalation and can cause pulmonary edema.
Source: quora.com
Phosphorus pentafluoride P F 5 is a phosphorus halide. Phosphorus pentafluoride Wikidata Phosphorus pentafluoride Wikipedia PPT Molecular Compounds Chapter 8 PowerPoint Lewis Structure What is the hybridization of the central phosphorus atom 188 Occurrence Preparation and Properties of Phosphorus Lewis Structure For Pf5 видео WikiBitme Exceptions to the Octet Rule ChemPaths The. The fluorine is in group 7 and has seven valence electrons. Back to Molecular Geometries Polarity Tutorial. It is toxic to inhale.
Source: quora.com
The electronegativity of fluorine is greater than that of phosphorusso the phosphorus atom is placed in the center of the molecule. It is a colourless toxic gas that fumes in air. PF5 Lewis StructureLewis Structure of PF5 Phosphorus PentafluorideDraw Lewis Structure for PF5. It is made up of one Phosphorus atom and five Fluorine atoms. Pf5 lewis structure phosphorus pentafluoride draw structures octet rule phosphorous obey chemistry expanded valence chem sulfur.
 Source: pngwing.com
Source: pngwing.com
Phosphorus pentafluoride which may be prepared by fiuorinating PC15 with AsF3 or CaF2 is molecular with a tbp structure in the solid state13 and under all other conditions. It is very toxic by inhalation and can cause pulmonary edema. It is a colourless toxic gas that fumes in air. The compound is nonpolar in nature because of the symmetric geometrical structure. A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot.
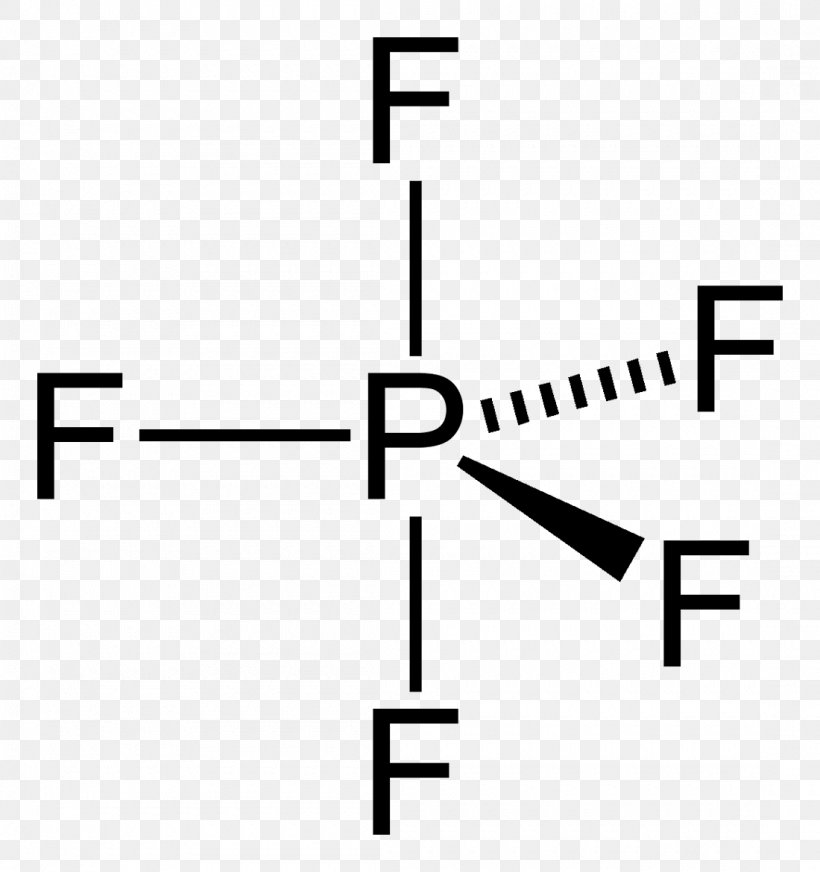 Source: favpng.com
Source: favpng.com
Molecular Geometry Polarity Tutorial. It is a colourless toxic gas that fumes in air. Phosphorus Pentafluoride on Wikipedia. To draw the PF5 Lewis structure follow the below instructions. The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution.
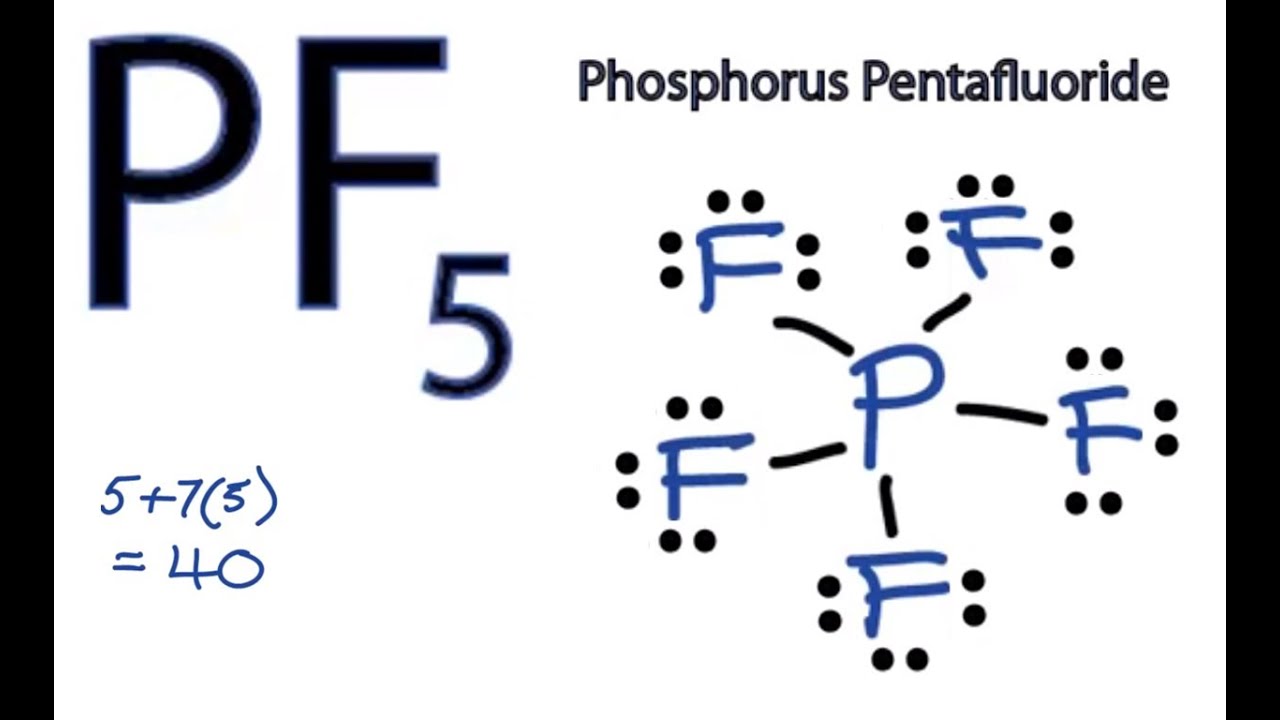 Source: youtube.com
Source: youtube.com
The phosphorous is in group 5 and has five valence electrons. Toxic and corrosive fumes are. But from PF5 formula it means that five fluorine so multiple 75 which. Phosphorus pentafluoride which may be prepared by fiuorinating PC15 with AsF3 or CaF2 is molecular with a tbp structure in the solid state13 and under all other conditions. To draw the PF5 Lewis structure follow the below instructions.
 Source: youtube.com
Source: youtube.com
Pf5 lewis structure phosphorus pentafluoride draw structures octet rule phosphorous obey chemistry expanded valence chem sulfur. It is a colourless toxic gas that fumes in air. It is toxic to inhale. The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution. The electronegativity of fluorine is greater than that of phosphorusso the phosphorus atom is placed in the center of the molecule.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title phosphorus pentafluoride lewis structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






