Po4 3 lewis structure
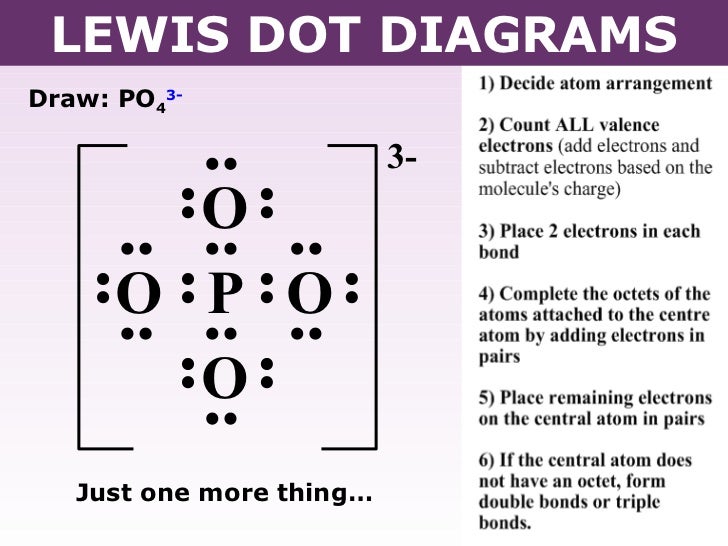
Po4 3 Lewis Structure. PO4 3- P O O O O 0 electrons remaining There are no electrons left to place DRAWING LEWIS STRUCTURES 23. Numbers next to atoms are their formal charges Starting at the upper right corner and moving clockwise we can make 4 resonance structures that expand the P octet to 10. The last resonance structure expands the P octet to 12 but is. You should check the formal charges to make sure that the total charge of the atom is -3.
 Write Lewis Structures For A Po43 B Ic4 C So32 D Hono Study Com From study.com
Write Lewis Structures For A Po43 B Ic4 C So32 D Hono Study Com From study.com
PO4 3- P O O O O 0 electrons remaining There are no electrons left to place DRAWING LEWIS STRUCTURES 23. Therefore the electron pair geometry is tetrahedral and the molecular geometry is tetrahedral. Write Lewis structures for. Zirconium Oxide with Calcium Impurity. PO4 3- P O O O O Now all atoms have a full octet DRAWING LEWIS STRUCTURES 24. Since its an ion you need to put brackets around the Lewis structure and then put a -3 outside.
MgAl 2 O 4 Spinel.
Also each oxygen atom has a -1 charge. Quiz your students on PO4 3- Lewis Structure With Formal Charge Resonance Molecular Geometry Shape Bond Angle using our fun classroom quiz game Quizalize and personalize your teaching. And all the four oxygen atoms are kept surrounding it. Lets do the Lewis structure for PO4 3-. PO4 3- P O O O O ions must be drawn with square brackets indicating the charge 3- DRAWING LEWIS STRUCTURES 25. PO4 3- Lewis Structure.
 Source: techiescientist.com
Source: techiescientist.com
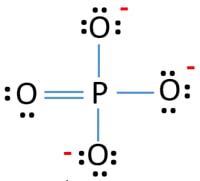
Name for Ca3PO42 and Lewis Structure Lewis Structure SO4 -2 Po4 3 Lewis StructureThese pictures of this page are aboutPO4 2 Lewis Structure How to Calculate the Formal Charges for PO4 3- Phosphate ion Tang 05 lewis dot diagrams Diagrama de Lewis del ion fosfato PO42- PO3 3- Lewis Structure How to Draw the Lewis Structure for PO33- SO4 2- Molecular Geometry Shape and. In the Lewis structure of PO43- there are a total of 32 valence electrons. The last resonance structure expands the P octet to 12 but is. PO4 3- Lewis Structure. Lets do the Lewis structure for PO4 3-.
 Source: clutchprep.com
Source: clutchprep.com
Since its an ion you need to put brackets around the Lewis structure and then put a -3 outside. MgAl 2 O 4 Spinel. Lets do the Lewis structure for PO4 3-. This negative 3 up here means we have three additional electrons. Also each oxygen atom has a -1 charge.
 Source: cms.gutow.uwosh.edu
Source: cms.gutow.uwosh.edu
PO4 3- P O O O O Now all atoms have a full octet DRAWING LEWIS STRUCTURES 24. You should check the formal charges to make sure that the total charge of the atom is -3. Calcium Carbide CaC 2. Quiz your students on PO4 3- Lewis Structure With Formal Charge Resonance Molecular Geometry Shape Bond Angle using our fun classroom quiz game Quizalize and personalize your teaching. Remember PO4 3- has a negative three charge on the molecule.
 Source: chemistryscl.com
Source: chemistryscl.com
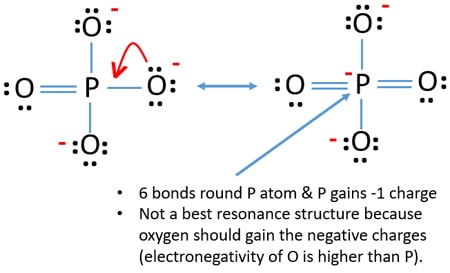
In the lewis structure of H 3 PO 4 there is one double between phosphorous atom and one oxygeen atom. Lets do the Lewis structure for PO4 3-. CaTiO 3 Perovskite. Numbers next to atoms are their formal charges Starting at the upper right corner and moving clockwise we can make 4 resonance structures that expand the P octet to 10. PO4 3- Lewis Structure.
 Source: study.com
Source: study.com
Remember PO4 3- has a negative three charge on the molecule. Rest of all bonds are single bonds. PO4 3- P O O O O ions must be drawn with square brackets indicating the charge 3- DRAWING LEWIS STRUCTURES 25. Related lewis structures to H 3 PO 4. PO4 3- Lewis Structure.
 Source: youtube.com
Source: youtube.com
Name for Ca3PO42 and Lewis Structure Lewis Structure SO4 -2 Po4 3 Lewis StructureThese pictures of this page are aboutPO4 2 Lewis Structure How to Calculate the Formal Charges for PO4 3- Phosphate ion Tang 05 lewis dot diagrams Diagrama de Lewis del ion fosfato PO42- PO3 3- Lewis Structure How to Draw the Lewis Structure for PO33- SO4 2- Molecular Geometry Shape and. Calcium Carbonate CaCO 3 Polymorphs. Lets do the Lewis structure for PO4 3-. That oxygen atom is connected to the phosphorous atom by a double bond has two lone pairs in its last shell. Lets do the Lewis structure for PO4 3-.
 Source: pinterest.com
Source: pinterest.com
Lets do the Lewis structure for PO4 3-. Inorganic salts of phosphoric acid. You should check the formal charges to make sure that the total charge of the atom is -3. Zirconium Oxide with Calcium Impurity. Between other oxygen atoms there are only single bonds with phosphorous atom.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Laboratory Chemical Safety Summary LCSS Datasheet. Write Lewis structures for. Silicon Sulfide SiS 2. Total electron pairs used to form bonds are determined by dividing the number of total valence electrons by two. PO4 3- P O O O O Now all atoms have a full octet DRAWING LEWIS STRUCTURES 24.
 Source: youtube.com
Source: youtube.com
Zirconium Oxide with Calcium Impurity. Calcium Carbide CaC 2. PO4 3- Lewis structure In the lewis structure of PO43- phosphorus is the central atom as it is the least electronegative. PO4 3- P O O O O 3. Write Lewis structures for.
 Source: youtube.com
Source: youtube.com
Between other oxygen atoms there are only single bonds with phosphorous atom. In the lewis structure of PO 43- three is a double bond between phosphorous atom and one oxygen atom. Numbers next to atoms are their formal charges Starting at the upper right corner and moving clockwise we can make 4 resonance structures that expand the P octet to 10. Step 8 in determining the Lewis Structure of PO43- PO 4 3-Step 8 Picture so Far. You should check the formal charges to make sure that the total charge of the atom is -3.
 Source: biochemhelp.com
Source: biochemhelp.com
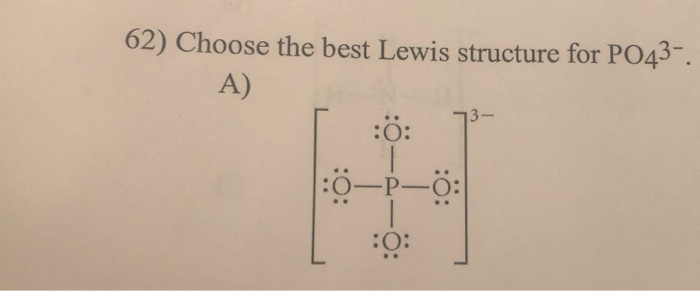
For the Lewis structure for PO4 3- you should take formal charges into account to find the best Lewis structure for the molecule. Phosphate 3- is a phosphate ion that is the conjugate base of hydrogenphosphate. How to Draw the Lewis Structure for PO4 3-. Calcium Carbide CaC 2. Ammonium Chloride NH 4 Cl Cu 3 Au Auricupride.
 Source: chemistryscl.com
Source: chemistryscl.com
Ammonium Chloride NH 4 Cl Cu 3 Au Auricupride. Calcium Carbide CaC 2. Ammonium Chloride NH 4 Cl Cu 3 Au Auricupride. In the lewis structure of H 3 PO 4 there is one double between phosphorous atom and one oxygeen atom. ReO 3 Rhenium trioxide.
 Source: study.com
Source: study.com
Calcium Carbide CaC 2. It is a phosphate ion and a trivalent inorganic anion. Oxygen has 6 weve got 4 Oxygens. Also each oxygen atom has a -1 charge. Medical Subject Headings MeSH.
 Source: techiescientist.com
Source: techiescientist.com
Also each oxygen atom has a -1 charge. Write Lewis structures for. Ammonium Chloride NH 4 Cl Cu 3 Au Auricupride. CaTiO 3 Perovskite. Also there are no lone pairs on phosphorous atom.
 Source: chegg.com
Source: chegg.com
PO4 3- Lewis Structure. Ammonium Chloride NH 4 Cl Cu 3 Au Auricupride. Since its an ion you need to put brackets around the Lewis structure and then put a -3 outside. Laboratory Chemical Safety Summary LCSS Datasheet. Oxygen has 6 weve got 4 Oxygens.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title po4 3 lewis structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.