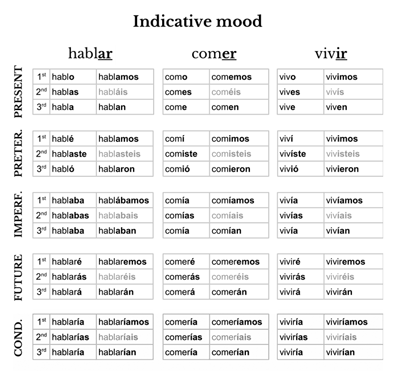
Sf5cl polar or nonpolar
Sf5cl Polar Or Nonpolar. Back to Molecular Geometries Polarity Tutorial. Ill tell you the polar or nonpolar list below. Follow asked Jan 12 18 at 1644. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.
 Wn Sf5cl Polar Or Nonpolar Lewis Structure Molecular Geometry Hybridization Bond Angle From wn.com
Wn Sf5cl Polar Or Nonpolar Lewis Structure Molecular Geometry Hybridization Bond Angle From wn.com
Images for Sf5cl. 85 2 2 silver badges 5 5. Therefore SF5Cl is polar. Quiz your students on SF5- Lewis Dot Strucutre - Polar or Nonpolar Bond Angle Hybridization Molecular Geometry using our fun classroom quiz game Quizalize and personalize your teaching. Follow asked Jan 12 18 at 1644. Explanation - - SF3Cl3 shows sp3d2 hybridization.
Therefore SF 5 Cl is polar.
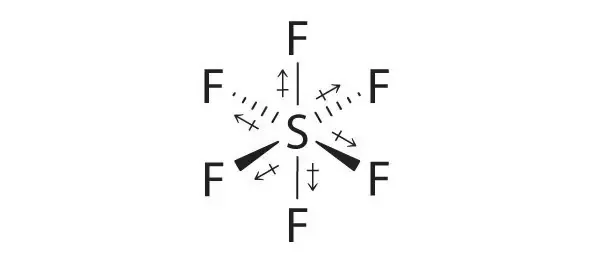
A polar molecule with two or more polar bonds must have an. Sulfur Hexafluoride on Wikipeida. - SF3Cl3 is polar molecule with S pulling electron clouds towards it. The molecular geometry of SF5Cl is octahedral and the charge distribution on the central atom is asymmetric. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. How many electron groups are there around the central atom.
 Source: study.com
Source: study.com
Is sf5cl polar or nonpolar. State whether the following molecules are polar or non-polar. The Shapes of Molecules Any bond between atoms of different elements will be polar as a result of the. Is sf5cl polar or nonpolar. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
 Source: wn.com
Source: wn.com
Since it is trigonal pyramidal it is asymmetrical and the vector sum of the bond dipoles is nonzero. List molecules polar and non polar. How many atoms are bonded to the central atom. By examining the lewis structure one can observe a dipole from the sulfur and oxygen. Sulfur dichloride is a cherry red liquid substance having a pungent smell.
 Source: tutor-homework.com
Source: tutor-homework.com
How many electron groups are there around the central atom. Refer to image for lewis structure. CAS No13780-57-9Sulfur chloridefluoride SClF5 OC-6. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. There are polar bonds present and I assumed that it is non polar because of its molecular geometry.
 Source: wn.com
Source: wn.com
An introduction to molecular symmetry of its symmetry CO2 is non-polar. Is SF5 polar or nonpolar. Refer to image for lewis structure. Sulfur dichloride is a cherry red liquid substance having a pungent smell. Follow asked Jan 12 18 at 1644.
 Source: theurbanbangla.com
Source: theurbanbangla.com
However The molecule itself is not entirely symmetrical unless Im thinking of symmetry incorrectly. Polar molecules Non-polar molecules etc Moderators. If its polar can you show me the bond dipoles and which direction the net dipole Is going. Secondly the difference between the electronegativity of sulfur and chlorine atoms makes the S-Cl bonds polar and as a result the entire molecule also becomes polar and gives a net dipole moment of 054D. Determine if the molecular is polar or non-polar - a molecule is i non-polar if the charge distribution is symmetric and ii polar if the charge distribution is asymmetric not symmetric.
 Source: theurbanbangla.com
Source: theurbanbangla.com
Therefore SF5Cl is polar. Thu Aug 04 2011 853 pm Has upvoted. Therefore SF5Cl is polarThis is a somewhat rare molecule but you can find a little info on it here. Determine if the molecular is polar or non-polar - a molecule is i non-polar if the charge distribution is symmetric and ii polar if the charge distribution is asymmetric not symmetric. 2 posts Page 1 of 1.
 Source: slidetodoc.com
Source: slidetodoc.com
H 2 N 2 O 2 Cl 2 These are truly nonpolar. Post by Chem_Mod Wed Sep 07 2011 739 am. What are the bond angles in the molecule. Examples of nonpolar molecules include. List molecules polar and non polar.
 Source: quora.com
Source: quora.com
Polar molecules Non-polar molecules etc Moderators. CAS No13780-57-9Sulfur chloridefluoride SClF5 OC-6. Ill tell you the polar or nonpolar list below. Follow asked Jan 12 18 at 1644. Lewis structure of XeOF2 - - XeO2F2 shows sp3d hybridization with seesaw structure.
 Source: wn.com
Source: wn.com
- SF3Cl3 is polar molecule with S pulling electron clouds towards it. Determine if the molecular is polar or non-polar - a molecule is i non-polar if the charge distribution is symmetric and ii polar if the charge distribution is asymmetric not symmetric. The Shapes of Molecules Any bond between atoms of different elements will be polar as a result of the. SF3Cl3 is a polar molecule. Examples of nonpolar molecules include.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Polarity and bond angles of BF2Cl and CHCl3. Secondly the difference between the electronegativity of sulfur and chlorine atoms makes the S-Cl bonds polar and as a result the entire molecule also becomes polar and gives a net dipole moment of 054D. Is sf5cl polar or nonpolar. Nonpolar molecules also form when atoms sharing a polar bond arrange such that the electric charges cancel each other out. Sulfur dichloride is a cherry red liquid substance having a pungent smell.
 Source: youtube.com
Source: youtube.com
How many atoms are bonded to the central atom. - SF3Cl3 is polar molecule with S pulling electron clouds towards it. Determine if the molecular is polar or non-polar - a molecule is i non-polar if the charge distribution is symmetric and ii polar if the charge distribution is asymmetric not symmetric. - It contains Xe with 1 e- pair O with 2 e- pairs F with 3 e- pairs. Back to Molecular Geometries Polarity Tutorial.
 Source: slideplayer.com
Source: slideplayer.com
How would you analyze the polarity and bond angles of BF2Cl and CHCl3. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. List molecules polar and non polar. Sf5cl polar or nonpolar.
 Source: bengislife.com
Source: bengislife.com
Since it is trigonal pyramidal it is asymmetrical and the vector sum of the bond dipoles is nonzero. Therefore SF5Cl is polarThis is a somewhat rare molecule but you can find a little info on it here. Answer SO2Cl2 Sulfuryl chloride is Polar What is polar and non-polar. Lewis structure of XeOF2 - - XeO2F2 shows sp3d hybridization with seesaw structure. The dipoles do not cancel out.
 Source: wn.com
Source: wn.com
What are the bond angles in the molecule. A polar molecule with two or more polar bonds must. Therefore SF5Cl is polar. Posted on March 07 2016. Images for Sf5cl.
 Source: theurbanbangla.com
Source: theurbanbangla.com
Polarity and bond angles of BF2Cl and CHCl3. Does it have a resonance. Secondly the difference between the electronegativity of sulfur and chlorine atoms makes the S-Cl bonds polar and as a result the entire molecule also becomes polar and gives a net dipole moment of 054D. By examining the lewis structure one can observe a dipole from the sulfur and oxygen. 85 2 2 silver badges 5 5.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sf5cl polar or nonpolar by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.