So3 2 lewis structure
So3 2 Lewis Structure. Therefore the structure in Step 1 is a plausible Lewis structure. The Step-by-Step Way to Draw Castles and. 2 SO 3 N 2 O 5 NO 2 2 S 2 O 7 Oxidant. Complete the octet shells.
 Compare The Structures Of So3 To Pf3 And Explain Why They Have Different Molecular Shapes Socratic From socratic.org
Compare The Structures Of So3 To Pf3 And Explain Why They Have Different Molecular Shapes Socratic From socratic.org
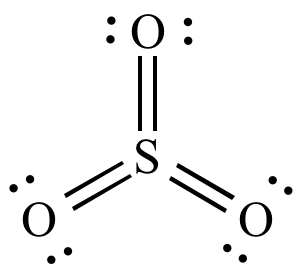
Calculate the formal charge for each atom in molecules A and B. Answer the following questions. READ THE NEW BOOK Draw 50 Buildings and Other Structures. Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. SO3 has 24 valence electrons. Lewis structure of so3.
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal.
0 electron which must be shared through pi bonding and unshared ie. Complete the octet shells. 5Distribution of other electron. Therefore P 6n 2 V 6 4 2 26 0 There are no π electrons in SO3-2. It is a conjugate base of a thiosulfate1-. Get your answers by asking now.
Source: quora.com
5Distribution of other electron. Here sulfur in the center because of its lowest electron capability and three oxygen around it. So3 2- lewis structure. Step 1 Figuring out the total number of valence electrons in the molecule is the first and most important step. There are no charges in sulfur atom.
 Source: meritnation.com
Source: meritnation.com
Complete the octet shells. So3 2- lewis structure. Therefore P 6n 2 V 6 4 2 26 0 There are no π electrons in SO3-2. 3Connect the atoms with single bonds. Here sulfur in the center because of its lowest electron capability and three oxygen around it.
 Source: youtube.com
Source: youtube.com
Is there are charges on sulfur atom in sulfite ion lewis structure. READ THE NEW BOOK Draw 50 Buildings and Other Structures. It is a conjugate base of a thiosulfate1-. SO3 2- Lewis Structure - How to Draw the Lewis Structure. So3 2- lewis structure.
 Source: chemistryscl.com
Source: chemistryscl.com
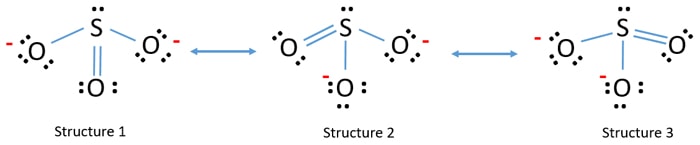
It is a conjugate base of a thiosulfate1-. S 0 -S2 -O. Lewis structure of so3. Lets do the SO3 2- Lewis structure. But there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2.
 Source: chemistryscl.com
Source: chemistryscl.com
5Distribution of other electron. 6 3 x 6 24The Lewis structure for SO 3 2-is requires you to place more than 8 valence electrons on Sulfur S. For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up herethere are two extra valence electrons. Step 1 Figuring out the total number of valence electrons in the molecule is the first and most important step. Sulfur brings 6 and oxygen brings 3 each.
 Source: youtube.com
Source: youtube.com
Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO32- ion. Answer the following questions. Put two electrons between the atoms to form chemical bonds. Determine the most stable Lewis structure and explain why. The Step-by-Step Way to Draw Castles and.
 Source: study.com
Source: study.com
4P6n2 -V 6x4 2 -26 26-26 0. SO3 has 24 valence electrons. There are three double bonds around sulfur atom with oxygen atoms in SO molecule. 0 electron which must be shared through pi bonding and unshared ie. Complete the octet shells.
Source: coursehero.com
Central atom of SO32- ion is sulfur. 6 3 x 6 24The Lewis structure for SO 3 2-is requires you to place more than 8 valence electrons on Sulfur S. So we have 26. Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. So3 2- lewis structure.
 Source: socratic.org
Source: socratic.org
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. In this Consider the Lewis structures of CF4 SO3 SO2 NF3 and OF2 which are given below. There are no charges in sulfur atom. Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal. Central atom of SO32- ion is sulfur.
 Source: chemistryscl.com
Source: chemistryscl.com
Two possible Lewis structures for the sulfur trioxide molecule SO3 molecule are shown in the figure below. Where V 6 6 6 6 -2 26 V is the number of valence electrons of the ion. Answer the following questions. Sulfur brings 6 and oxygen brings 3 each. 3Connect the atoms with single bonds.
 Source: youtube.com
Source: youtube.com
SO3 has 24 valence electrons. SO3 Molecular Geometry Lewis Structure and Polarity. Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO 3 2-ion. SO3 has 24 valence electrons. Put two electrons between the atoms to form chemical bonds.
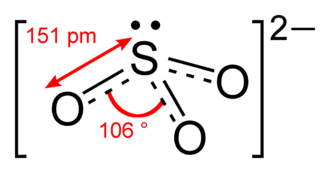 Source: socratic.org
Source: socratic.org
Step 1 Figuring out the total number of valence electrons in the molecule is the first and most important step. Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. Around sulfur atom there are four bonds and a single lone pair in the lewis structure of SO 3 2-ion. Therefore the structure in Step 1 is a plausible Lewis structure. The Step-by-Step Way to Draw Castles and.
 Source: youtube.com
Source: youtube.com
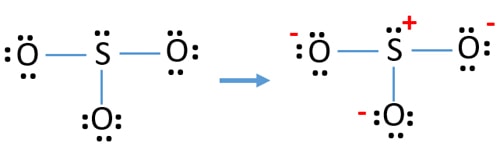
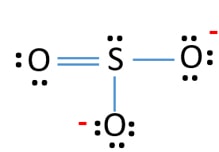
The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. Where n in this case is 4 since SO3-2 consists of four atoms. Where V 6 6 6 6 -2 26 V is the number of valence electrons of the ion. SO3 Molecular Geometry Lewis Structure and Polarity. Lewis Structure of SO3 Valence.
 Source: quora.com
Source: quora.com
Where V 6 6 6 6 -2 26 V is the number of valence electrons of the ion. For the SO3 2- compound we have 26 total valence electrons and that includes these two electrons up here–there are two extra valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. SO 3 has 24 valence electrons. Put two electrons between the atoms to form chemical bonds.
 Source: youtube.com
Source: youtube.com
Electrons are placed around each. SO3 Molecular Geometry Lewis Structure and Polarity. Complete the octet shells. Each oxygen atom has two lone pairs in SO 3 lewis structure. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title so3 2 lewis structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






