Williamson ether synthesis lab report
Williamson Ether Synthesis Lab Report. Lab work under the fumehood 4. Ethers are an important class of compounds that contain an oxygen atom which is doubled bonded to two organic groups. Mathew Fields 3-19-2011 Williamson Synthesis Purpose. 1 Draw both enantiomers of gauifenesin and indicate which is enantiomer is the.
![]() The Williamson Ether Synthesis Lab Report Docsity From docsity.com
The Williamson Ether Synthesis Lab Report Docsity From docsity.com
In this experiment -3-chloro-12-propane-diol will be allowed to react with the conjugate base of Guaicol 2-methoxyphenol via an S N2 reaction to provide guaifenesin as a racemic mixture. Simplest way to synthesize an ether is to have an alkoxide react with a primary haloalkane or a sulfonate ester under typical SN2 conditions. Mathew Fields 3-19-2011 Williamson Synthesis Purpose. Preparation Alkenes E1 And E2 Rxns Unsaturation Lab 9 Preparation of Alcohols. The Williamson Ether Synthesis A. The Williamson reaction is widely used in.
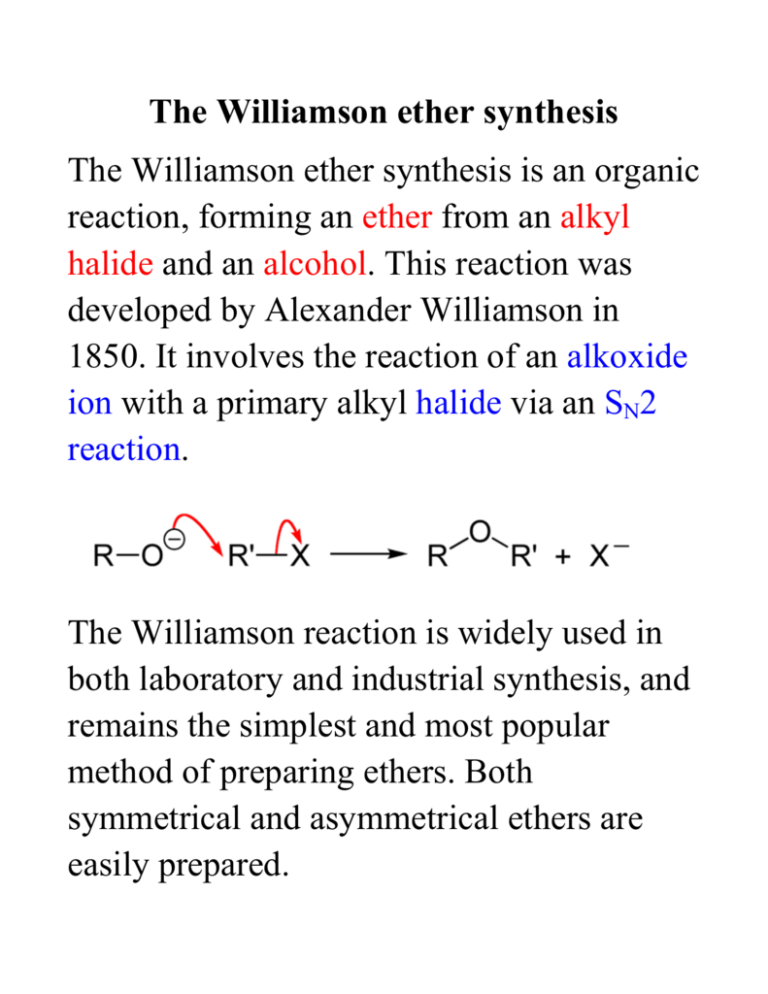
Ether is widely used in our daily life especial in medical field.
They are generally prepared by an Sn2 displacement of al alkoxide onto a primary or secondary alkyl halide. Ether is widely used in our daily life especial in medical field. Yes Data Entry Amount Of Reactant Used In Grams Amount Of Product Obtained In Grams Beginning Of Melting Point Range End Of Melting Point Range 0145 0154 1299 1331 22pts Calculations And Analysis. Williamson ether synthesis i have this assignment chegg com lab report diigo groups three reactions are depicted below indicate which propose a mechanism for the synth 10 preparation of phenacetin from acetaminophen studocu synthesis of triphenylmethanol quizlet Home. 1 Draw both enantiomers of gauifenesin and indicate which is enantiomer is the. The Williamson reaction is widely used in.

Williamson Synthesis Lab Report Nicholas Johnson Drawer. Simplest way to synthesize an ether is to have an alkoxide react with a primary haloalkane or a sulfonate ester under typical SN2 conditions. Ethers can be synthesized in standard S N 2 conditions by coupling an alkoxide with a haloalkanesulfonate ester. The mechanism begins with the base abstracting the proton from the alcohol to form an alkoxide intermediate. Pavia 71 71A 72 73 75 711 Smith Section 96 Pre-lab Questions.

Lab work under the fumehood 4. The second step occurs as an SN2 substitution reaction Robert J. Simplest way to synthesize an ether is to have an alkoxide react with a primary haloalkane or a sulfonate ester under typical SN2 conditions. Williamson ether synthesis i have this assignment chegg com lab report diigo groups three reactions are depicted below indicate which propose a mechanism for the synth 10 preparation of phenacetin from acetaminophen studocu synthesis of triphenylmethanol quizlet Home. Ethyl Ester Synthesis Lab Report.
 Source: docsity.com
Source: docsity.com
R OH R O. Mathew Fields 3-19-2011 Williamson Synthesis Purpose. Lab work under the fumehood 4. Simplest way to synthesize an ether is to have an alkoxide react with a primary haloalkane or a sulfonate ester under typical SN2 conditions. Since this reaction follows the Sn2 process it also has the same limitations.
 Source: studentshare.org
Source: studentshare.org
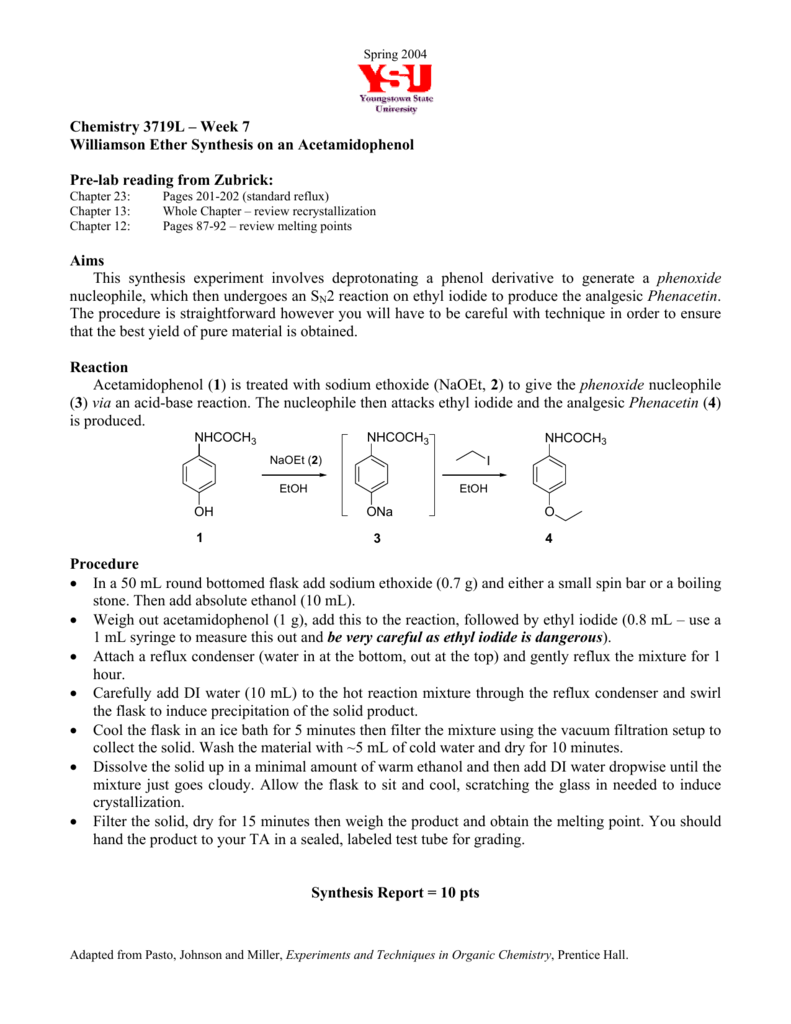
Williamson Ether Synthesis Data And Lab Report Submission 5pts Williamson Ether Synthesis Are You Completing This Experiment Online. One way to synthesize these types of compounds is through a Williamson ether synthesis which is. CHEM M52LBH52LB Experiment 2 Page 1 EXPERIMENT 1 WILLIAMSON ETHER SYNTHESIS OF GUAIFENESIN AND ISOLATION OF AN EXPECTORANT FROM COUGH TABLETS Reading Assignment. The Williamson Ether Synthesis A. They specifically target the nerve endings in the brain.
 Source: coursehero.com
Source: coursehero.com
When applied to an unsymmetrical ether as in this case there are two different combinations of reactants are possible. 11 in the 9th edition McMurry textbook. The same material will also be isolated from over the counter tablets via. Williamson Synthesis Lab Report Nicholas Johnson Drawer. With the theoretical and experimental yield obtained the percent yield was calculated and found to be 3636.
 Source: youtube.com
Source: youtube.com
Reduction of Fluorenone Recrystallization lab report Unknown compounds Lab 9 Pinacol Rearrangement. R L R O R alkoxide R is primary. Yes Data Entry Amount Of Reactant Used In Grams Amount Of Product Obtained In Grams Beginning Of Melting Point Range End Of Melting Point Range 0145 0154 1299 1331 22pts Calculations And Analysis. R OH R O. They are generally prepared by an Sn2 displacement of al alkoxide onto a primary or secondary alkyl halide.
![]() Source: docsity.com
Source: docsity.com
The Williamson ether synthesis. Ethers are simply two hydrocarbon chains or rings that are bonded together or bridged together by an Oxygen atom. Ethers are an important class of compounds that contain an oxygen atom which is doubled bonded to two organic groups. Since this reaction follows the Sn2 process it also has the same limitations. Reduction of Fluorenone Recrystallization lab report Unknown compounds Lab 9 Pinacol Rearrangement.
 Source: studylib.net
Source: studylib.net
When applied to an unsymmetrical ether as in this case there are two different combinations of reactants are possible. Ether is widely used in our daily life especial in medical field. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. The purpose of this lab was to Views 13 Downloads 0 File size 519KB. Williamson Ether Synthesis in its Simplest Form.
 Source: coursehero.com
Source: coursehero.com
Ethers can be synthesized in standard S N 2 conditions by coupling an alkoxide with a haloalkanesulfonate ester. Pavia 71 71A 72 73 75 711 Smith Section 96 Pre-lab Questions. Synthesis Lab Report Of Williamson Ethers. Hanna Thomson Lab 10 Erica Tuesday 8am The mechanism for step two the substitution portion of this reaction is shown below in figure 2. This reaction was developed by Alexander Williamson in 1850.

The theoretical yield for the reaction was 154 g and the experimental yield was 056g. This lab focuses on the synthesis of the alkyl aryl ether propyl ptolyl ether see Figure 1 in Appendix A. They are generally prepared by an Sn2 displacement of al alkoxide onto a primary or secondary alkyl halide. The Williamson ether synthesis yields an ether by the reaction of an alkyl halide with. The mechanism begins with the base abstracting the proton from the alcohol to form an alkoxide intermediate.
 Source: studylib.net
Source: studylib.net
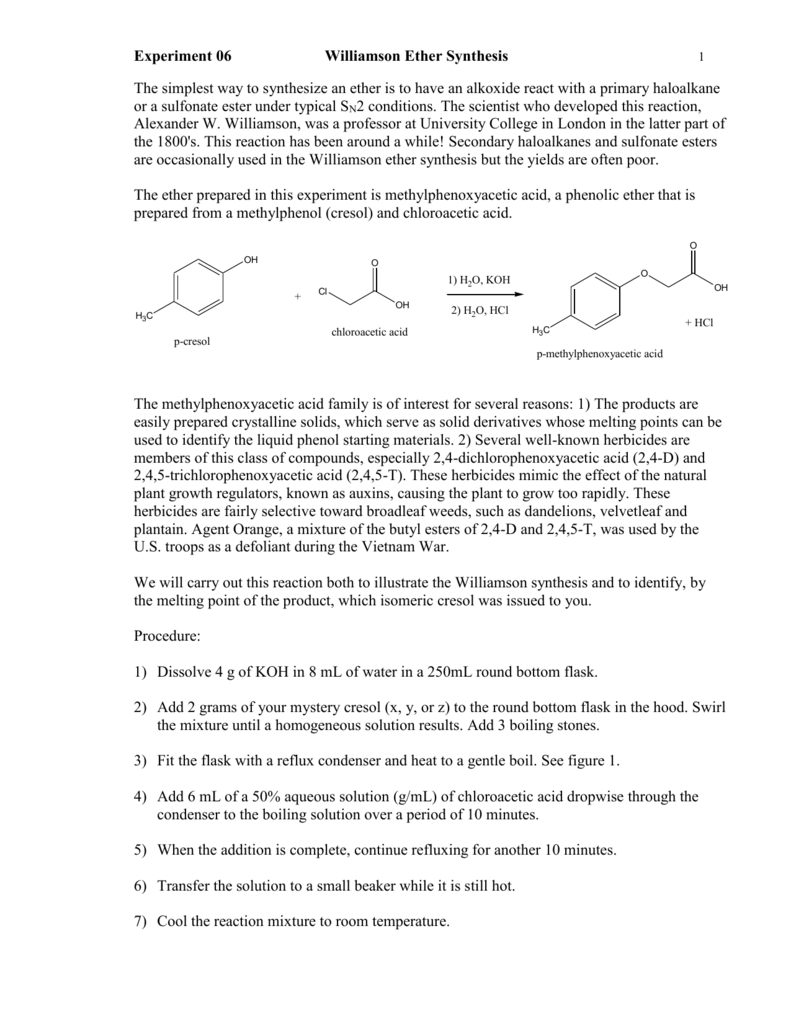
Synthesis Ether Williamson Of Report Lab. The Williamson reaction is widely used in. List three limitations of the Williamson ether synthesis and explain how our choice of reagents circumvents those limitations. Ether is widely used in our daily life especial in medical field. The Williamson Ether Synthesis can specifically be found in sections 17-2 and 18-2.
 Source: pdfslide.net
Source: pdfslide.net
Williamson Synthesis Lab Report Nicholas Johnson Drawer. One way to synthesize these types of compounds is through a Williamson ether synthesis which is. 1 Draw both enantiomers of gauifenesin and indicate which is enantiomer is the. Williamson Ether Synthesis - Chemistry. R OH R O.
 Source: studocu.com
Source: studocu.com
They are generally prepared by an Sn2 displacement of al alkoxide onto a primary or secondary alkyl halide. The second step occurs as an SN2 substitution reaction Robert J. Williamson Ether Synthesis Preparation of Phenacetin from Acetaminophen Organic Chem Lab 7. Martin Belmont 2015 4th Ed pp. Sn2 reaction from Williamson Ether Synthesis lab 10 in Organic Chemistry Lab Manual and Course Materials by Landrie McQuade and Yermolina.
 Source: studylib.net
Source: studylib.net
Synthesis Ether Williamson Of Report Lab. Phenacetin is a drug used for pain relief and a fever reducing agent however it was. Pavia 71 71A 72 73 75 711 Smith Section 96 Pre-lab Questions. Synthesis Lab Report Of Williamson Ethers. Williamson Synthesis Lab Report.

They are generally prepared by an Sn2 displacement of al alkoxide onto a primary or secondary alkyl halide. Ethers are an important class of compounds that contain an oxygen atom which is doubled bonded to two organic groups. Sn2 reaction from Williamson Ether Synthesis lab 10 in Organic Chemistry Lab Manual and Course Materials by Landrie McQuade and Yermolina. Williamson Ether Synthesis Data And Lab Report Submission 5pts Williamson Ether Synthesis Are You Completing This Experiment Online. In this experiment -3-chloro-12-propane-diol will be allowed to react with the conjugate base of Guaicol 2-methoxyphenol via an S N2 reaction to provide guaifenesin as a racemic mixture.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title williamson ether synthesis lab report by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.