Molecular shape of icl3
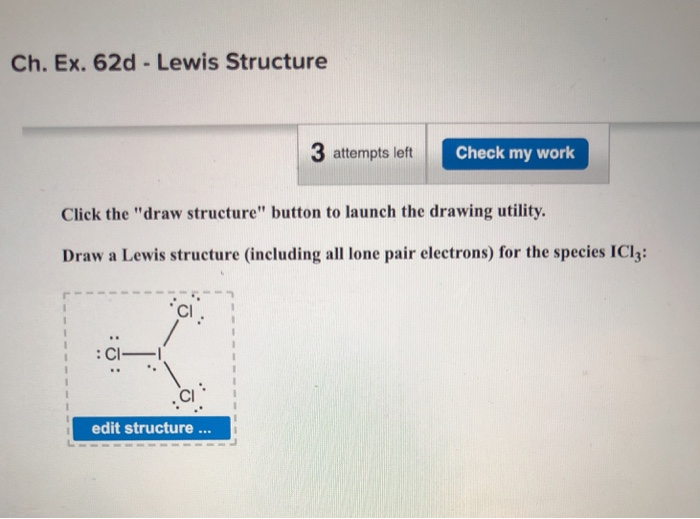
Molecular Shape Of Icl3. I C l3 a Lewis structure is first structure and has two extra lone pairs on the central atom. ICl3 has three bond pairs and two lone pairs of electrons. EN Cl-I 05. What is the electronic geometry of iodine trichloride ICl3.
 Icl3 Lewis Structure Molecular Geometry Novocom Top From novocom.top
Icl3 Lewis Structure Molecular Geometry Novocom Top From novocom.top
Iodine starts off with 7 electrons and each chlorine provides it with 1 giving a total of 5 pairs. An explanation of the molecular geometry for the ICl3 ion Iodine trichloride including a description of the ICl3 bond angles. Laboratory Chemical Safety Summary LCSS Datasheet. Molecules with an trigonal bipyramidal electron pair geometries have sp3d or dsp3 hybridization at the central atom. The six carbon-hydrogen bond orbitals in. Original post by zattyzatzat the back of my textbook says this shape is a trigonal planar.
Which one is it.
What is the electronic geometry of iodine trichloride ICl3. The back of my textbook says this shape is a trigonal planar. I C l3 a Lewis structure is first structure and has two extra lone pairs on the central atom. Both lone pairs of electrons occupy the equatorial positions to achieve stability and minimize bond pair lone pair repulsions. The electron geometry will be trigonal bipyramidal. Original post by zattyzatzat the back of my textbook says this shape is a trigonal planar.
 Source: chegg.com
Source: chegg.com
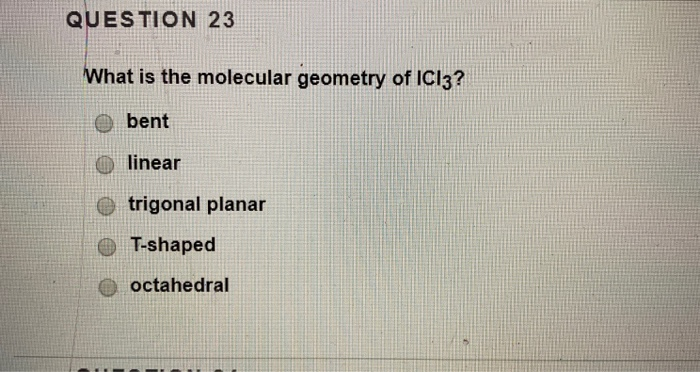
What is the electronic geometry of iodine trichloride ICl3. They are very shape specific. The geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape. New Icl3 Molecular Geometry Image. I C l3 a Lewis structure is first structure and has two extra lone pairs on the central atom.
 Source: youtube.com
Source: youtube.com
The back of my textbook says this shape is a trigonal planar. They are very shape specific. Original post by zattyzatzat the back of my textbook says this shape is a trigonal planar. Molecules with an trigonal bipyramidal electron pair geometries have sp3d or dsp3 hybridization at the central atom. These five electron pairs arrange themselves into a trigonal bipyramidal orientation.
 Source: techiescientist.com
Source: techiescientist.com
The ICl3 molecule will have a T-shaped molecular geometry. Shape is determined by the relative placement of the. ICl3 has three bond pairs and two lone pairs of electrons. What is the molecular shape of ICl3. It includes the general shape of the molecule as well as bond lengths bond angles torsional angles and any other geometrical parameters that.
 Source: enotes.com
Source: enotes.com
Which one is it. I know that this makes a T-shape due to the two unbonded pairs and the CL-I-Cl bond is slightly less than 90 degrees but why is Cl not all. New Icl3 Molecular Geometry Image. An explanation of the molecular geometry for the ICl3 ion Iodine trichloride including a description of the ICl3 bond angles. The electron geometry for the.
 Source: thoughtco.com
Source: thoughtco.com
Original post by zattyzatzat the back of my textbook says this shape is a trigonal planar. These five electron pairs arrange themselves into a trigonal bipyramidal orientation. If youre AX5 your electronic geometry is trigonal bipyramidal. Option 2 would be our. But when I check online its says its a T-Shape molecule.
 Source: clutchprep.com
Source: clutchprep.com
Both lone pairs of electrons occupy the equatorial positions to achieve stability and minimize bond pair lone pair repulsions. Both lone pairs of electrons occupy the equatorial positions to achieve stability and minimize bond pair lone pair repulsions. An explanation of the molecular geometry for the ICl3 ion Iodine trichloride including a description of the ICl3 bond angles. A trigonal planar b trigonal pyramid c trigonal bipyramid d t-shaped e octahedron. VSEPR Theory - Iodine Trichloride ICl3 - Expanded.
 Source: novocom.top
Source: novocom.top
What is the electron geometry and molecular geometry shape of. I C l3 a Lewis structure is first structure and has two extra lone pairs on the central atom. Therefore it has a total of 3 bond pairs and two lone pairs. The ICl3 molecule will have a T-shaped molecular geometry. A 1 g of an element give A 2 g of its oxide.
 Source: chegg.com
Source: chegg.com
A 1 g of an element give A 2 g of its oxide. If youre AX5 your electronic geometry is trigonal bipyramidal. EN Cl-I 05. I am confused on why the Cl in the trigonal bipyramidal of ICl3 are on the axis and has one in the trigonal planar. Option 2 would be our.
 Source: youtube.com
Source: youtube.com
The electron geometry will be trigonal bipyramidal. Answer to Draw the lewis structure for ICl3 iodine trichloride. Molecules with an trigonal bipyramidal electron pair geometries have sp3d or dsp3 hybridization at the central atom. Iodine starts off with 7 electrons and each chlorine provides it with 1 giving a total of 5 pairs. I know that this makes a T-shape due to the two unbonded pairs and the CL-I-Cl bond is slightly less than 90 degrees but why is Cl not all.
 Source: techiescientist.com
Source: techiescientist.com
Iodine starts off with 7 electrons and each chlorine provides it with 1 giving a total of 5 pairs. ICl3 a Lewis structure is first structure and has two extra lone pairs on the central atom b VSEPR 3 bp 2 lp 5 shape is trigonal bipyramidal c Molecular shape is T shaped second structure. Molecular geometry is trigonal planar. A sample of peanut oil weighing. Homework question 49 asks for the shape of ICL3 and the ClICl bond angle.
 Source: thoughtco.com
Source: thoughtco.com
VSEPR Theory - Iodine Trichloride ICl3 - Expanded. Get What Is The Molecular Geometry Of Icl3 Pictures. Which one is it. After saponification is complete 846 ml of 0273 M. Iodine chloride ICl3 UNII-1E5KQ66TRQ.
Source: thestudentroom.co.uk
I C l3 a Lewis structure is first structure and has two extra lone pairs on the central atom. 1576 g is added to 25 ml of 0421 M KOH. I know that this makes a T-shape due to the two unbonded pairs and the CL-I-Cl bond is slightly less than 90 degrees but why is Cl not all. The electron geometry will be trigonal bipyramidal. Homework question 49 asks for the shape of ICL3 and the ClICl bond angle.
 Source: transtutors.com
Source: transtutors.com
1576 g is added to 25 ml of 0421 M KOH. The electron geometry for the. When describing molecular geometry we deduce the shape by looking at the distribution of electrons around the central atom including not just atoms but also lone electron pairs. A trigonal planar b trigonal pyramid c trigonal bipyramid d t-shaped e octahedron. VSEPR Theory - Iodine Trichloride ICl3 - Expanded.
Source:
After saponification is complete 846 ml of 0273 M. An explanation of the molecular geometry for the ICl3 ion Iodine trichloride including a description of the ICl3 bond angles. New Icl3 Molecular Geometry Image. A sample of peanut oil weighing. I C l3 a Lewis structure is first structure and has two extra lone pairs on the central atom.
 Source: techiescientist.com
Source: techiescientist.com
Homework question 49 asks for the shape of ICL3 and the ClICl bond angle. Molecules with an trigonal bipyramidal electron pair geometries have sp3d or dsp3 hybridization at the central atom. What is the electronic geometry of iodine trichloride ICl3. Which one is it. Laboratory Chemical Safety Summary LCSS Datasheet.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title molecular shape of icl3 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.